Abstract
Backgrounds
Interleukin-6 (IL-6) blockers including tocilizumab and sarilumab were approved by the U.S. Food and Drug Administration (FDA) in June 2021 for the treatment of patients with moderate to severe COVID-19. The use of sarilumab or tocilizumab in COVID-19 patients has been related to a reduction in mortality compared to standard care. Recent evidence has emerged concerning drug-induced liver injury (DILI) after sarilumab or tocilizumab applications in COVID-19 patients.
Aims
The study aimed to estimate DILI associated with sarilumab or tocilizumab in treating moderate to severe patients infected with SARS-Cov-2.
Methods
We conducted a retrospective pharmacovigilance study by data mining of the FDA’s adverse event reporting systems (FAERS) database from the first quarter of 2004 to the fourth quarter of 2021 in confirmed COVID-19 patients. We analyzed DILI cases associated with tocilizumab or sarilumab in treating COVID-19 patients from the FAERS during this period. Disproportionality analysis and Bayesian analysis of COVID-19 patients were utilized for case analysis, and we also next compared the onset time and fatality rates of DILI following tocilizumab or sarilumab.
Results
A total of 275 cases of TCZ or SAR-related DILI reports were extracted. A total of 192 AEs cases were related to tocilizumab (TCZ), and 83 were related to sarilumab (SAR). In patients treated with TCZ, most were < 75 years old (51.57%), with more male than female (46.35% vs. 13.02%). The correlation between IL-6 receptor antagonists and DILI was stronger in SAR (ROR = 12.94; 95%CI 9.6–17.44) than in TCZ (ROR = 1.33; 95%CI 1.14–1.55). The onset time of DILI was different between TCZ and SAR, and a significant difference was observed in TCZ than SAR (P < 0.0001). A significant difference was observed in the mortality rate of TCZ and SAR (P = 0.0009). DILI associated with COVID-19 patients treated with TCZ appeared to have earlier onset-time (1(0–46) day) VS. SAR (3.5(0–27) day).
Conclusion
This study shows strict monitor ought to be paid for TCZ or SAR when used for COVID-19 patients with poor liver function.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-022-07896-0.
Keywords: Coronavirus disease 2019 (COVID-19), Tocilizumab, Sarilumab, Drug Induced Liver Injury, FAERS, Epidemiology
Introduction
At the end of 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged as a new human pathogen. This unknown virus, which causes coronavirus disease 2019 (COVID-19), was named by the WHO on February 11, 2020. On March 11, 2020, the WHO declared COVID-19 a global pandemic causing enormous economic and social disruption worldwide. As of March 20, 2022, there were more than 468 million confirmed cases of COVID-19 worldwide, including approximately 6 million deaths [1]. Severe cytokine release syndrome (CRS) is a life-threatening acute systemic inflammatory response characterized by multiple organ failure and fever. It plays a central role in the pathogenesis of SARS-CoV-2 infection. SARS-CoV-2 infection induces dose- and time-dependent production of cytokines. In severe COVID-19 disease, elevated levels of cytokines, especially interleukin 6 (IL-6), have been observed and were considered a critical factor in inflammation [2, 3]. COVID-19 patients with elevated IL-6 levels and evidence of hyperinflammation had increased rates of more severe symptoms [4, 5]. These clinical findings can provide new ideas for the treatment of COVID-19.
Various options have been used to treat COVID-19, such as remdesivir, lopinavir/ritonavir, umifenovir, convalescent plasma, SAR, TCZ, inhalational budesonide, methylprednisolone, hydroxychloroquine, azithromycin, and mesenchymal stem cells [6]. IL-6 blockers can significantly reduce the risk of death and mechanical ventilation in moderate to severe patients compared to other treatments while also causing fewer serious adverse effects [7, 8]. IL-6 blockers are classified as anti-IL-6 receptor monoclonal antibodies (SAR, TCZ) (Table 1) or anti-IL-6 monoclonal antibodies (siltuximab, olokizumab). Some studies show a potentially clinically significant impact of TCZ on lung function preservation [9, 10]. TCZ (molecular weight is 148KDa) and SAR (molecular weight is 150KDa) are humanized monoclonal antibodies that bind to membrane-bound and soluble IL-6 receptors to inhibit the IL-6 signalling pathway by blocking dimerization of glycoprotein (gp)130 molecules on the cell membrane [11–13]. In a population pharmacokinetic study observing 1793 RA patients, the patients received a 1-h infusion of TCZ 4 or 8 mg/kg every 4 weeks for 24 weeks. In the population pharmacokinetic (PK) analysis, a dose proportional increas in maximum concentration (Cmax) was observed after TCZ 4 or 8 mg/kg every 4 weeks, however, the increasing in the area under the concentration–time (AUC) curve and the through concentration was more than dose proportional. Ohsugi et al. found that in vivo, sIL-6R saturation maximum (> 90%) when serum TCZ concentrations were > 1ug/ml [14, 15]. Compared with TCZ, the binding kinetics and functional activity of SAR were more higher and SAR bound to IL-6R with higher affinity than TCZ [16]. In a PK characterization of SAR in patients with RA including subcutaneous SAR 150 mg or 200 mg every 2 weeks for up to one year, a greater than dose-proportional increase in SAR exposure was observed [14].
Table 1.
Summary of FDA-approved IL-6 receptor antagonist
| Generic name | Brand name | Indication | Year of approval by FDA |
|---|---|---|---|
| Tocilizumab | Actemra | RA, CRS, sJIA, Giant cell arteritis, COVID-19 | 2003; tocilizumab was granted an EUA for the treatment of COVID-19 in June 2021 |
| Sarilumab | Kevzara | RA | 2017 |
RA rheumatoid arthritis, CRS cytokine release syndrome, sJIA systemic juvenile idiopathic arthritis
IL-6 receptor antagonists are used to treat some hyperinflammatory diseases, such as rheumatoid arthritis [17], giant cell arteritis [18], and cytokine release syndrome (CRS) induced by chimeric antigen receptor (CAR) T-cell therapy [19]. COVID-19 can attack the immune system, causing it to over-react and produce dangerous levels of inflammatory factors [20]. Therefore, IL-6 receptor antagonists have been used to treat severe COVID-19. In a randomized clinical trial (RCT) conducted in England involving 452 hospitalized patients with severe COVID-19, the use of TCZ did not result in a significantly better prognosis or lower mortality than placebo at 28 days (19.7% in the TCZ and 19.4% in the placebo groups) [21]. In another RCT involving 389 patients with COVID-19 who were not receiving mechanical ventilation, TCZ reduced the probability of progression of mechanical ventilation or death compared to the placebo group (12.0% in the TCZ and 19.3% in the placebo groups) [22].
Adverse events (AEs) of TCZ in treating non-COVID diseases are upper respiratory tract infection, urinary tract infection, nasopharyngitis, hypercholesterolemia, and neutropenia. Hypertension and gastrointestinal perforation are also reported [23]. In patients with severe or critical COVID-19, AEs were reported in 43% of patients who received TCZ compared to 34% who did not (P = 0.26) [24]. A phase III clinical trial in India showed that 36% of patients in the TCZ group and 25% of patients in the standard care group had AEs. Serious AEs occurred 20% in the TCZ group and 17% in the standard care group. In this study, involving 180 patients, 33 of 91(36%) patients in the TCZ group and 22 0f 89(25%) patients in the standard care group had AEs; 18 of 91(20%) and 15 of 89(17%) had serious AEs; 13 of 91(14%) and 15 of 89(17%) patients died during the study [25].
Currently, there is no systematic retrospective analysis that summarizes the AEs of IL-6 receptor antagonists in treating patients with COVID-19. Our study aimed to use the US Food and Drug Administration Adverse Event Reporting System (FAERS) to analyse and compare the differences and links of DILI in COVID-19 patients receiving TCZ or SAR to supply a reference for clinicians to treat COVID-19. We further examined the onset-time and fatality and hospitalization rates for DILI of COVID-19 patients treated with TCZ or SAR.
Method
Data source
The FAERS database, a publicly available spontaneous reporting system, is typically used for initial investigations of new or rare AEs and for evaluating drug-drug interactions. The database contains detailed information on the demographics of medications, indications, outcomes, and sources of reports. All AEs were coded using Preferred Terms (PTs) in the Medical Dictionary for Regulatory Activities (MedDRA, version 25.0) Terminology. The MedDRA hierarchy contains five levels, system organ class (SOC), high level group term (HLGT), high level term (HLT), preferred term (PT), lowest level term (LLT). The “System Organ Classes,” grouped by disease etiology, manifestation site, or purpose, are the high-tier group terms. PTs, which describe disease symptoms or diagnosis, indications, laboratory results, and procedures, are low-level group terms used in the FAERS database [6].
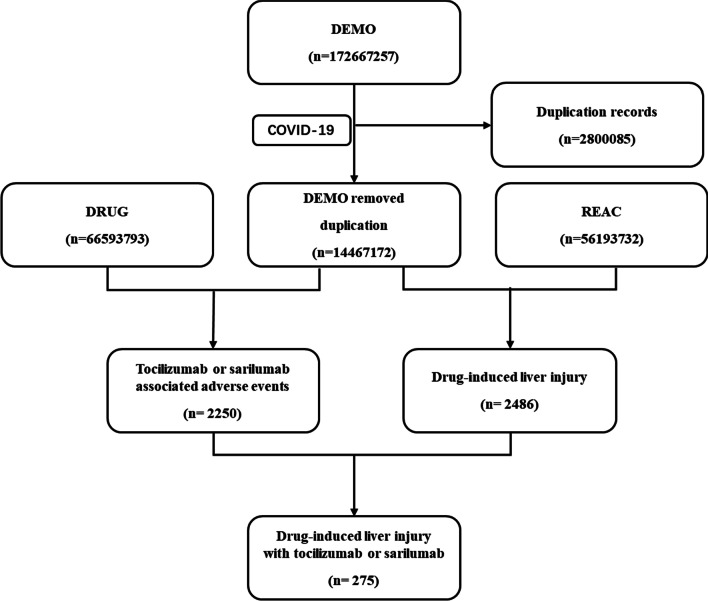
Data were extracted from the FAERS database from the first quarter of 2004 to the fourth quarter of 2021 and we obtained a total of 17,267,257 reports from the FEARS database (Fig. 1). From these data, we extracted COVID-19 cases from the second quarter of 2020 to the fourth quarter of 2021. We removed the duplicate records according to FDA’ recommendations, and we finally identify 192 cases of DILI associating with TCZ as a suspected drug, and 83 cases by SAR.
Fig. 1.
Process of the selection of cases of IL-6 receptor antagonists-associated liver injury from the FAERS database. DEMO demographic information, DRUG drug information, REAC adverse events
Identification of coronavirus infection
We searched the case reports of COVID-19 patients with DILI after treatment with TCZ or SAR, from the second quarter of 2020 to the fourth quarter of 2021. During the search, we use keywords as: COVID-19, 2019-Ncov, SARS-Cov-2.
Drug identification and adverse drug event classification
Drug-induced liver injury is based on the Preferred Term of the MedDRA, and we searched the FAERS database to confirm COVID-19 patients cases treated with TCZ (also called actemra, atlizumab, roactemra) and SAR (also called Kevzara). We focus on adverse effects in COVID-19 patients treated with TCZ or SAR. In this study, we examined TCZ and SAR according to the COVID-19 treatment guidelines and considered DILI was associated with these two drugs. The MedDRA preferred terms used to interpret hepatotoxicity cases of TCZ and SAR are shown in Table 2.
Table 2.
MedDRA preferred terms that used to retrieve adverse event cases of IL-6 receptor antagonists in FAERS
| PT (preferred terms) |
|---|
| LIVER INJURY |
| LIVER DAMAGE |
| HEPATIC DAMAGE |
| HEPATOTOXICITY |
| HEPATITIS |
| NONALCOHOLIC FATTY LIVER DISEASE |
| LIVER FATTY INFILTRATION |
| CHOLESTASIS |
| BILIARY CHOLANGITIS |
| HEPATIC ENCEPHALOPATHY |
| HEPATIC FAILURE |
| PORTAL HYPERTENSION |
| HEPATIC NECROSIS |
| DILI |
| HEPATOCELLULAR INJURY |
| HEPATIC ENZYME ABNORMAL |
| BLOOD BILIRUBIN ABNORMAL |
Data mining and statistical analysis
Data mining algorithms detect FAERS signals, where a signal means a statistical association between drugs and drug-associated adverse events [11]. These algorithms consist of the proportional reporting ratio (PRR), the reporting odds ratio (ROR), the information component (IC), and the empirical Bayes geometric mean (EBGM) [26]. PRR and ROR are non-Bayesian disproportionality analyses, whereas the IC and EBGM are Bayesian analyses. A two-by-two contingency table was constructed to identify signals of reported event counts for TCZ and SAR (Table 3).
Table 3.
A two-by-two contingency table for a drug-adverse event combination
| With an adverse event of interest | Other adverse events | Total | |
|---|---|---|---|
| With a drug of interest | a | b | (a + b) |
| Without a drug of interest | c | d | (c + d) |
| Total | (a + c) | (b + d) | (a + b + c + d) |
PRR = (a/[a + b])/(c/[c + d]) (criteria: PRR ≥ 2,χ2 ≥ 4, n ≥ 3); ROR = (a/c)/(b/d), 95% CI = eln(ROR) – 1.96(1/a+1/b+1/c+1/d)^0.5 (criteria: 95% CI > 1, n ≥ 2); IC = log2a*(a + b + c + d)/([a + b][a + c]), IC025 = eln(IC)–1.96(1/a+1/b+1/c+1/d)^0.5 (criteria:IC025 > 0); EBGM = a*(a + b + c + d)/([a + b][a + c]), EBGM05 = eln(EBGM)–1.64(1/a+1/b+1/c+1/d)^0.5 (criteria: EBGM05 > 2, n > 0). CI confidence interval, n indicates the number of co-occurrences, χ2 chi-squared, IC information component, IC025 the lower limit of the 95% two-sided CI of the IC, EBGM05 the lower limit of the 90% one-sided CI of the EBGM
We also estimated the onset time of DILI with TCZ or SAR in COVID-19 patients, defined as time interval between the onset-time of DILI and the date of initiation of the medication, and we removed entered erroneously (Reports with EVENT_DT earlier than START_DT) or inaccurate date entries. In addition, we analysed the reports of fatal events in COVID-19 patients due to DILI, which were calculated by dividing the number of fatal events by the total number of TCZ- or SAR-associated DILI events.
GraphPad Prism 8.0.2(GraphPad Software, CA, USA) was used to statistically analyse the DILI of COVID-19 patients treated with TCZ or SAR. The two-tailed Student's t-test was used to analyse between-group analysis differences, and the difference between multiple groups was investigated by the Kruskal–Wallis test. P < 0.05 was considered statistically significant.
Results
Descriptive analysis
From May 2020 to December 2021, the FAERS database had 2486 adverse events reports in COVID-19 patients treated with monoclonal antibody. Among these cases, we filtered out 192 cases with suspected TCZ-associated DILI and 83 cases with suspected SAR-associated DILI. From the analysis results, we found that the IL-6 receptor antagonists used for the treatment of COVID-19 were mainly TCZ and SAR. Table 4 summarizes the clinical characteristics of these patients. These cases were concentrated in 2021. 80.73% of the adverse reactions related to DILI reported by TCZ for COVID-19 treatment were from patients in North America and Europe, while 91.56% of SAR were from North America and Europe. For the patient age, eliminating unknown data, adverse reactions associating with DILI are mainly concentrated between the ages of 45–64, respectively TCZ 30.21% and SAR 3.61%. Surprisingly, 96.39% of cases on SAR-related DILI were reported without mentioning age. Further, the AEs of these two monoclonal antibodies were more in females than in males (TCZ vs. SAR, 46.35% vs. 2.41%), excluding unknown data, meanwhile, 96.39% of cases on SAR-related DILI were reported without regard to gender. Additionally, SAR-related DILI cases were mainly uploaded by physicians (98.8%), and TCZ-related cases were uploaded by pharmacists (27.08%) and physicians (33.33%). And no SAR-related DILI cases have been reported to FAERS database after the first quarter of 2021 (Additional file 1).
Table 4.
Clinical characteristics of COVID-19 patients with IL-6 receptor antagonists-associated DILI sourced from the FAERS database (2020q2 to 2021q4)
| Characteristics | Drug report, n (%) | |
|---|---|---|
| TCZ | SAR | |
| Patient age (year) | ||
| 18–44 | 20 (10.42%) | 0 (0.00%) |
| 45–64 | 58 (30.21%) | 3 (3.61%) |
| 65–74 | 21 (10.94%) | 0 (0.00%) |
| 75–84 | 11 (5.73%) | 0 (0.00%) |
| ≥ 85 | 2 (1.04%) | 0 (0.00%) |
| Unknown | 80 (41.67%) | 80 (96.39%) |
| Patient gender | ||
| Female | 25 (13.02%) | 1 (1.20%) |
| Male | 89 (46.35%) | 2 (2.41%) |
| Unknown | 78 (40.63%) | 80 (96.39%) |
| Reporter occupation | ||
| Consumer | 2 (1.04%) | 0 (0.00%) |
| Pharmacist | 52 (27.08%) | 1 (1.20%) |
| Physician | 64 (33.33%) | 82 (98.80%) |
| Unknown | 74 (38.54%) | 0 (0.00%) |
| Report time | ||
| 2020Q2 | 13 (6.77%) | 6 (7.23%) |
| 2020Q3 | 29 (15.10%) | 13 (15.66%) |
| 2020Q4 | 23 (11.98%) | 61 (73.49%) |
| 2021Q1 | 20 (10.42%) | 3 (3.61%) |
| 2021Q2 | 53 (27.60%) | 0 (0.00%) |
| 2021Q3 | 25 (13.02%) | 0 (0.00%) |
| 2021Q4 | 29 (15.10%) | 0 (0.00%) |
| Area | ||
| Africa | 2 (1.04%) | 0 (0.00%) |
| Asian | 22 (11.46%) | 0 (0.00%) |
| Europe | 102 (53.13%) | 13 (15.66%) |
| North America | 53 (27.60%) | 63 (75.90%) |
| South America | 13 (6.77%) | 7 (8.43%) |
| Outcomes | ||
| Congenital anomaly | 0 (0.00%) | 1 (1.22%) |
| Death | 30 (17.14%) | 30 (36.59%) |
| Disability | 1 (0.57%) | 1 (1.22%) |
| Hospitalization initial or prolonged | 27 (15.43%) | 37 (45.12%) |
| Life-threatening | 6 (3.42%) | 29 (35.37%) |
| Other serious (important medical event) | 137 (78.29%) | 54 (65.85%) |
| Required intervention to prevent permanent impairment | 2 (1.14%) | 0 (0.00%) |
Disproportionality analysis and Bayesian analysis
The data showed a strong association between TCZ, SAR, and DILI. Intraclass analysis of the correlation between IL-6 receptor antagonist and DILI showed the following ranking: SAR (ROR = 12.94; 95%CI 9.6–17.44) > TCZ (ROR = 1.33; 95%CI 1.14–1.55) (Table 5). Notably, SAR has more strong association with DILI than TCZ because of its stronger signal.
Table 5.
The association of TCZ and SAR with DILI
| Drugs | N | ROR | PRR | IC | EBGM |
|---|---|---|---|---|---|
| (95% two-sided CI) | (χ2) | IC025 | EBGM05 | ||
| TCZ | 191 | 1.33 (1.14, 1.55) | 1.3 (13.27) | 0.36 (0.31) | 1.28 (1.13) |
| SAR | 83 | 12.94 (9.6, 17.44)* | 7.31 (468.91)* | 2.83 (2.1)* | 7.11 (5.54)* |
TCZ-associated DILI ROR=1.33, 95% two-sided CI(1.14,1.55), PRR=1.3, χ2=13.27, EBGM=1.28, 95% one-sided CI=1.2; SAR-associated DILI ROR=12.94, 95% two-sided CI(9.6,17.44), PRR=7.31, χ2=468.91, EBGM=7.11, 95% one-sided CI=5.54; * means a strong signal that represents a strong statistical association between the drug and the adverse reaction
Onset times of IL-6 receptor antagonists-associated DILI
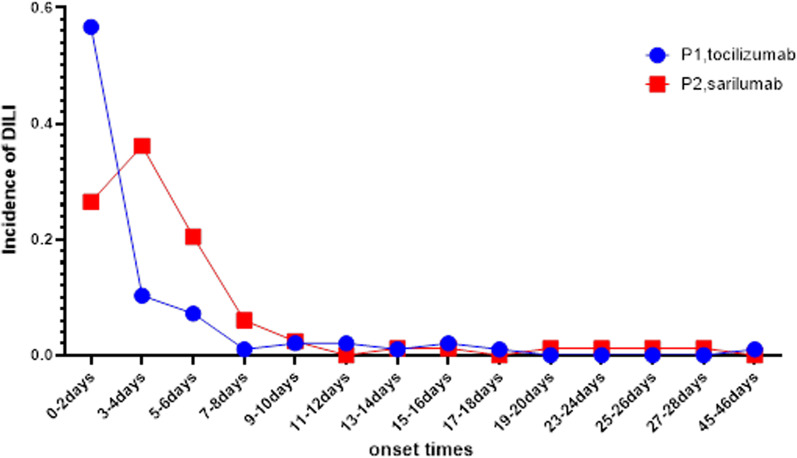
The median onset time of the DILI for TCZ is 1(IQR 0–46) day and SAR is 3.5(IQR 0–27) days. The onset time of DILI was different between these two IL-6 inhibitors. Significant differences were observed for TCZ versus SAR (two-tailed Student's t-test, P < 0.05). There is also a noticeable difference in the peak onset times of adverse reactions between the two drugs (Fig. 2). According to the data, TCZ-related DILI accounted for 67.01% the first four days after receiving TCZ therapies, and SAR-associated DILI accounted for 83.13% the first six day after receiving SAR therapies. Notably, we found that DILI may occur after the first dose of TCZ in treating COVID-19, whereas SAR showed a peak after the last dose, which may due to different compositions and structures result to different pharmacokinetic (PK) and pharmacodynamic (PD).
Fig. 2.
The difference in the peak onset times of adverse reactions between TCZ and SAR
Outcomes of TCZ and SAR
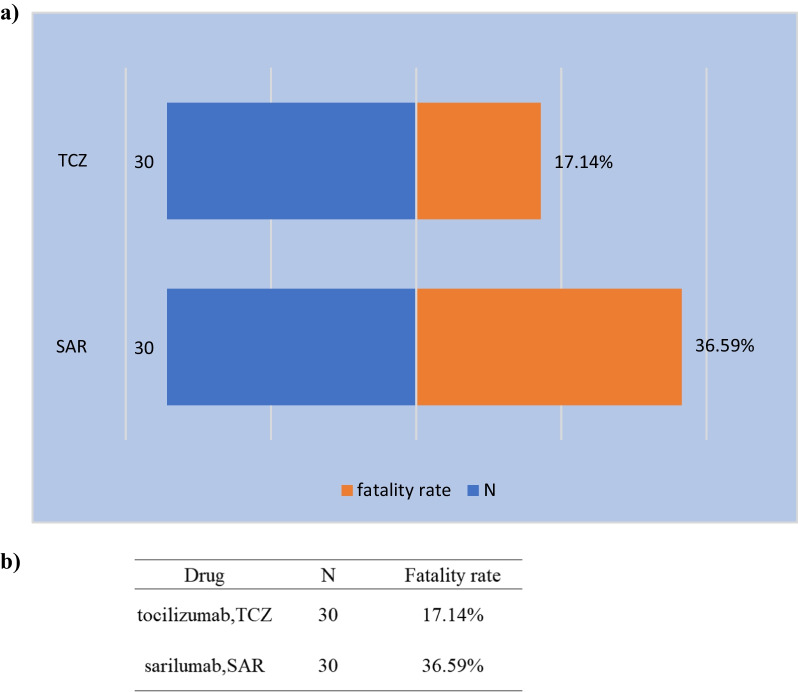
To analyse the prognosis of patients with COVID-19 treated with IL-6 receptor antagonists, we calculated the proportions of outcomes (death, disability, initial or prolonged hospitalization, life-threatening, and other serious conditions requiring interventions) after receiving TCZ or SAR therapies, and the results are shown in Table 6. Among COVID-19 patients treated with TCZ,17.14% of patients died, and 15.4% were hospitalized compared with SAR, 36.6% died, 45% hospitalized. Additionally, there was a significant difference in the mortality rate of TCZ and SAR (P = 0.0009). The mortality rates for these two drugs are shown in Fig. 3. Despite the significant difference in death proportions, we could not be certain that receiving TCZ or SAR was associated with mortality in COVID-patients, the reason is that, in the FEARS database, the sample capacity of COVID-19 patients treated with TCZ and SAR is not consistent.
Table 6.
The outcomes of TCZ and SAR
| Outcome | TCZ (N, %) | SAR (N, %) |
|---|---|---|
| Congenital anomaly | 0 (0.00) | 1 (0.01) |
| Death | 30 (0.17) | 30 (0.37) |
| Disability | 1 (0.01) | 1 (0.01) |
| Hospitalization-initial or prolonged | 27 (0.15) | 37 (0.45) |
| Life-threatening | 6 (0.03) | 29 (0.35) |
| Other serious/important medical events | 137 (0.78) | 54 (0.66) |
| Required intervention to prevent permanent impairment/damage | 2 (0.01) | 0 (0.00) |
Fig. 3.
The number of reports and fatality rate for DILI following TCZ or SAR therapy
Discussion
Infection by SARS-CoV-2 induces a dose-dependent production of IL-6 from bronchial epithelial cells [27]. Elevated IL-6 levels are associated with poor prognosis in COVID-19, including respiratory failure and death. Furthermore, IL-6 plays a critical role, not only in metabolic function but, in the regulation of multiple physiological systems, including tissue homeostasis such as liver regeneration [28]. The liver is an important organ for metabolic activities, and abnormal liver function will be accompanied by an increase in the level of transaminase [29]. IL-6 receptor blockers neutralize membrane-bound and soluble forms of the IL-6 receptor, blocking the activation of cytokines and regulation of the immune response to infection. IL-6 receptor blockers are monoclonal antibodies approved for use in rheumatoid arthritis (RA) by FDA, respectively TCZ in 2013 and SAR 2017. Based on a review of the data from RECOVERY clinical trial (NCT #04381936), the COVACTA clinical trial (NCT #04320615), the EMPACTA clinical trial (NCT#04372186) and the REMDACTA clinical trial (NCT #04409262), FDA believed that TCZ may be effective for the treatment of COVID-19 patients and approved it for the treatment of COVID-19 in June, 2021. WHO strongly recommend treatment with IL-6 receptor antagonist for patients with severe or critical COVID-19 [30]. COVID-19 treatment guidelines recommended SAR may be used as an alternative if TCZ is not available because it has demonstrated a similar clinical benefit in improving survival and reducing the duration of organ support in the REMAP-CAP trial [31].
In this disproportionality analysis of FAERS database, we detected signals of increased risks of DILI associated with TCZ versus SAR in patients with COVID-19. Our work is a collection until recently to compare the associations, onset-time, prognosis of COVID-19 patients with DILI after using TCZ or SAR in the real-world based on the FAERS database. In this study, we noticed that TCZ or SAR-related DILI affected more man than woman. Eliminating unknown data, we found that 46.35% of the COVID-19 patients who received TCZ therapy were male and 13.02% were female, which is similar to the gender composition of patients in other RCTs, with more male than female participants [32]. TCZ was initially approved by the FDA for the treatment of RA, which is more common in middle-aged women, but more men than women have AEs among COVID-19 patients treated with TCZ [33, 34]. We may conclude that TCZ treatment of the COVID-19 patients is might be related to more AEs because the drugs population was dominated by female patients, but the AEs after medication were dominated by males. Mao et al. reported that the incidence of liver injury in male patients with COVID-19 is higher than that in female patients [35], but whether it is related to TCZ treatment remains to be further verified. It is a pity that 96.39% of the COVID-19 patients who received SAR therapy were not reported gender in FAERS database, so there is insufficient evidence for analysing the connection between SAR-associated DILI and gender.
We also noticed that among the COVID-19 patients, except for the unknown data, among the patients receiving TCZ treatment, the elderly over 65 years old accounted for 17.71%, and in the SAR, the proportion of the elderly over 65 years old was 0; surprisingly, 96.39% of the COVID-19 patients who received SAR therapy were also not reported age which is similar with gender report. A RECOVERY clinical trial [36] in the United Kingdom, participants’ mean age is 64 years-old. Among the reporters, except for the unknown data, for the COVID-19 patients receiving TCZ treatment, consumers accounted for 1.04%, pharmacists accounted for 27.08%, and physicians accounted for 33.33%; while for the COVID-19 patients receiving SAR treatment, no consumer reported data, and pharmacists accounted for 1.20%, physicians accounted for 98.8%. SAR treated the COVID-19 patients as an alternative if TCZ is not available because the evidence of efficacy for TCZ is more extensive than for SAR. In terms of reporting time, the proportion of SAR reported in Q4 in 2020 is as high as 73.49%; after 2021Q1, the proportion of SAR adverse reactions reported is 0. Compared to SAR, DILI caused by TCZ has the highest reporting rate in 2021Q2, reaching 27.6%, which probably because FDA issued an Emergency Use Authorizations (EUA) for TCZ for the treatment of COVID-19 in hospitalized adults and pediatric patients [37].
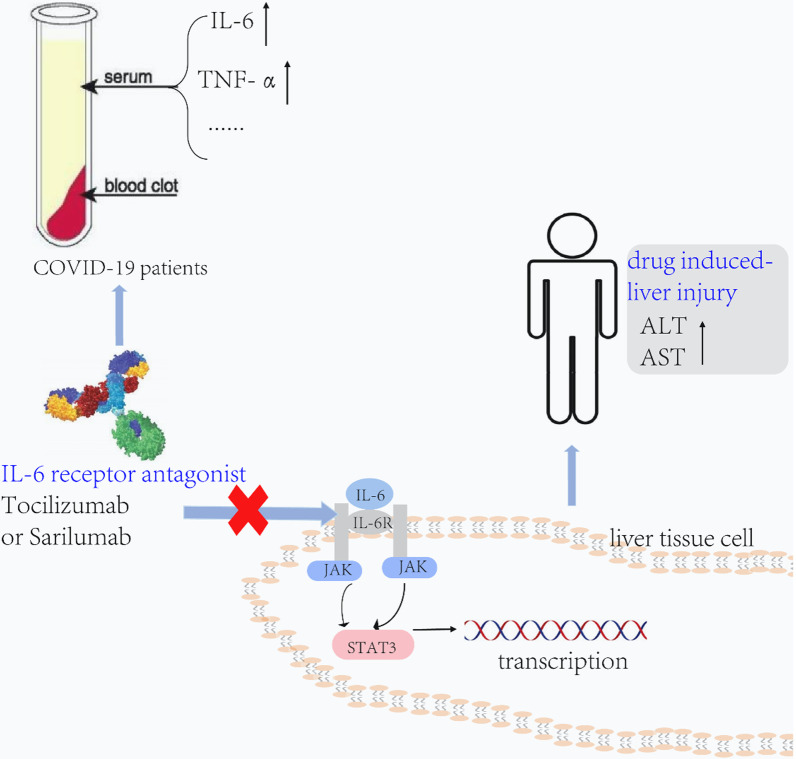
Deaths accounted for 17.14% of the 192 cases of DILI associated with TCZ; among the 83 COVID-19 patients treated with SAR, deaths accounted for 36.59%. Many factors can affect the pharmacokinetics of IL-6 inhibitors, such as age, weight, body surface area, comorbidities, and treatment with other drugs. AUC0-14 day doubled when the SAR usage dose increased from 150 mg/kg (every two weeks) to 200 mg/kg (every two weeks) [38]. The clearance of TCZ is concentration-dependent, related to body surface area, and its half-life increases with dose and frequency of administration [39]. In this study, we highlighted the effects of COVID-19 patient age and gender on the prognosis of IL-6 receptor antagonist treatment in the FAERS database. We found that SAR (ROR = 12.94; 95% two-sided CI 9.6–17.44) was more strongly associated with DILI than TCZ (ROR = 1.33; 95% two-sided CI1.14–1.55). The results are consistent with a previous systematic review finding that TCZ has a more uncertain association with DILI than SAR [32]. The primary laboratory abnormalities reported with TCZ treatment are elevated liver enzyme levels that appear to be dose-dependent [23]. Muhovic et al. also reported a 52-year-old patient with COVID-19 who was healthy in the past. On the second day after receiving TCZ treatment, ALT increased from normal level to 1541U/L, AST increased from normal level to 1076U/L, and a few days after drug withdrawal, liver function returns, which shows that TCZ treatment can lead to liver damage in COVID-19 patients [40]. According to past reports, the effect of TCZ on liver function may be related to blocking IL-6. IL-6-TAK-STAT3 pathway is a significant signal for liver regeneration, and the binding of IL-6 to its receptor can activate the JAK/STAT3 signalling pathway to promote hepatocyte proliferation [41, 42]. TCZ inhibits the binding of IL-6 to its receptor to block the JAK/STAT3 signalling pathway, which induces severe DILI by promoting hepatocyte apoptosis and inhibiting liver regeneration [43] (Fig. 4).
Fig. 4.
Mechanism of liver injury caused by IL-6 receptor antagonist therapy for COVID-19 patients
For pregnant women, there are insufficient data to determine whether there is a TCZ or SAR-associated risk for major birth defects or miscarriage. mAbs are actively transported across the placenta as pregnancy progresses, and this may affect immune responses in the exposed fetus. Given the lacking of data, current recommendations advise against the use of IL-6 receptor antagonists during pregnancy [44]. There are no systematic observational or randomized controlled trial data on the effectiveness of TCZ or SAR for the treatment of acute COVID-19 in pediatric patients. So there is insufficient evidence for the Panel to recommend either for or against the use of TCZ or SAR in hospitalized children with COVID-19.
Data from COVID-19 patients obtained from the FAERS database in this study showed that ARs occurred mostly within two days after TCZ treatment compared to four days after SAR treatment. The onset time difference also reached statistical significance (P = 0.0292). In previous research, IL-6 returned to baseline levels on day 14 after a subcutaneous injection of low-dose SAR and on day 20 after a subcutaneous injection of low-dose TCZ. The peak time for IL-6R after SAR treatment was earlier than for TCZ, ranging from 11–13 days for SAR and 19–22 days for TCZ [45]. In addition, we also found that the proportion of COVID-19 patients who died in ARs reports after SAR therapies were significantly higher than that of TCZ, which may explain why clinicians are more willing to use TCZ than SAR in patients with severe COVID-19.
Our study has the following limitations: (1) the FAERS database has incomplete information such as sex, age, and reporting area, and (2) this is a retrospective study based on spontaneous submitting data, so we could not assess the causality between DILI and TCZ with SAR, and (3) confounding factors are difficult to control, for example, if COVOD-19 patients also received other drug treatments when receiving TCZ or SAR treatment, and if the patient had other primary diseases that could affect the occurrence of DILI.
Conclusion
IL-6 inhibitors are associated with the occurrence of AEs related to TCZ and SAR. We profiled DILI associated with the application of TCZ or SAR in COVID-19 patients with clinical characteristics, onset time and prognosis, based on the FAERS database. Clinicians should strictly monitor this potential DILI when treating COVID-19 patients with IL-6 receptor antagonists.
Supplementary Information
Additional file 1. Information on COVID-19 patients treated with TCZ or SAR obtained from the FEARS database.
Acknowledgements
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. GQ, YXD and TBY analysed and interpreted data, plotted figures, and wrote the manuscript draft. WJS, ZB and CJY participated in the interpretation of data. LZL designed and directed the research. LZL and YQL assisted in preparing this manuscript and providing constructive suggestions.
Funding
Shanghai "Rising Stars of Medical Talent" Youth Development Program “Outstanding Youth Medical Talent” (No.SHWSRS(2021)_099); Shanghai Talent Development Funding (No. 2020110); National Natural Science Foundation of China Youth Science Foundation Project (No. 81603199).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html#collapse2
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There are no competing interests for any authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qian Gao and Xuedong Yin equally contributed to this work
Contributor Information
Qiaoling Yang, Email: yangql@shchildren.com.cn.
Zhiling Li, Email: lizhiling22@163.com.
References
- 1.Coronarvirus Resource Center, Johns Hopkins University Medicine. 2022. Johns Hopkins University Medicine. Medicine JHU. https://coronavirus.jhu.edu/map.html. Accessed 22 Mar 2020.
- 2.Kang S, et al. Historical overview of the interleukin-6 family cytokine. J Exp Med. 2020 doi: 10.1084/jem.20190347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JS, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316. doi: 10.7150/thno.49713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Available from: https://covid.aiims.edu/therapeutic-options-for-acute-covid-19-infection-in-humans/.
- 7.Xu X, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizaldy Taslim Pinzona VOW, Buana RB. Interleukin-6 (IL-6) inhibitors as therapeutic agents for coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Infect Public Health, 2021. [DOI] [PMC free article] [PubMed]
- 9.Khanna D, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387(10038):2630–2640. doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]
- 10.Khanna, D. Efficacy and safety of tocilizumab for the treatment of systemic sclerosis: results from a phase 3 randomized controlled trial. in 2018 ACR/ARHP Annual Meeting. 2018. ACR.
- 11.Bate A, Evans S. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–436. doi: 10.1002/pds.1742. [DOI] [PubMed] [Google Scholar]
- 12.Sakaeda T, et al. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10(7):796–803. doi: 10.7150/ijms.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felsenstein S, et al. COVID-19: immunology and treatment options. Clin Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Medicines Agency. RoActemra (tocilizumab): summary of product characteristics. http://www.emea.europa.eu/humandocs/PDFs/EPAR/RoActemra/H-955-PI-en.pdf. Accessed 23 Mar 2009.
- 15.Ohsugi Y, Kishimoto T. The recombinant humanized antiIL-6 receptor antibody tocilizumab, an innovative drug for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2008;8(5):669–681. doi: 10.1517/14712598.8.5.669. [DOI] [PubMed] [Google Scholar]
- 16.Potocky T, Rafique A, Fairhurst J, et al. Evaluation of the binding kinetics and functional bioassay activity of sarilumab and tocilizumab to the human il-6 receptor alpha (il-6ra) [abstract no.ICW-C15–3] Mod Rheumatol. 2016;26(Suppl):S69–70. [Google Scholar]
- 17.Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77(17):1865–1879. doi: 10.1007/s40265-017-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, Rech J. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377(4):317–328. doi: 10.1056/NEJMoa1613849. [DOI] [PubMed] [Google Scholar]
- 19.Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15(8):813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhry D, Singh PK. Tocilizumab and COVID-19. Indian J Crit Care Med. 2020;24(9):741. doi: 10.5005/jp-journals-10071-23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charan J, Dutta S, Kaur R, Bhardwaj P, Sharma P, Ambwani S, et al. Tocilizumab in COVID-19: a study of adverse drug events reported in the WHO database. Expert Opin Drug Saf. 2021;20(9):1125–1136. doi: 10.1080/14740338.2021.1946513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veiga VC, Prats JA, Farias DL, Rosa RG, Dourado LK, Zampieri FG, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021; 372. [DOI] [PMC free article] [PubMed]
- 25.Soin AS, Kumar K, Choudhary NS, Sharma P, Mehta Y, Kataria S, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021;9(5):511–521. doi: 10.1016/S2213-2600(21)00081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10(7):796–803. doi: 10.7150/ijms.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshikawa T, Hill T, Li K, Peters CJ, Tseng C-TK. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83(7):3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17(6):395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 29.Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2015;17(1):14. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal A, Rochwerg B, Lamontagne F, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for COVID-19. BMJ. 2020 doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 31.Derde LP, Investigators R-C. Effectiveness of tocilizumab, sarilumab, and anakinra for critically ill patients with COVID-19 the REMAP-CAP COVID-19 immune modulation therapy domain randomized clinical trial. MedRxiv. 2021.
- 32.Ghosn L, Chaimani A, Evrenoglou T, Davidson M, Graña C, Schmucker C, et al. Interleukin‐6 blocking agents for treating COVID‐19: a living systematic review. Cochrane Database Syst Rev. 2021;(3). [DOI] [PMC free article] [PubMed]
- 33.Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a Synopsis. Am J Manag Care. 2014;20(7 Suppl):S128–135. [PubMed] [Google Scholar]
- 34.Nelson JL, Østensen M. Pregnancy and rheumatoid arthritis. Rheum Dis Clin N Am. 1997;23(1):195–212. doi: 10.1016/S0889-857X(05)70323-9. [DOI] [PubMed] [Google Scholar]
- 35.Mao R, Qiu Y, He J-S, Tan J-Y, Li X-H, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horby PW, Pessoa-Amorim G, Peto L, Brightling CE, Sarkar R, Thomas K, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. MedRxiv. 2021.
- 37.Genentech tocilizumab letter of Authority June 24 2021. https://www.fda.gov/media/150319/download.
- 38.Xu C, Su Y, Paccaly A, Kanamaluru V. Population pharmacokinetics of sarilumab in patients with rheumatoid arthritis. Clin Pharmacokinet. 2019;58(11):1455–1467. doi: 10.1007/s40262-019-00765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frey N, Grange S, Woodworth T. Population pharmacokinetic analysis of tocilizumab in patients with rheumatoid arthritis. J Clin Pharmacol. 2010;50(7):754–766. doi: 10.1177/0091270009350623. [DOI] [PubMed] [Google Scholar]
- 40.Muhović D, Bojović J, Bulatović A, Vukčević B, Ratković M, Lazović R, Smolović B. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40(8):1901–1905. doi: 10.1111/liv.14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.da Silva CG, Studer P, Skroch M, Mahiou J, Minussi DC, Peterson CR, et al. A20 promotes liver regeneration by decreasing SOCS3 expression to enhance IL-6/STAT3 proliferative signals. Hepatology. 2013;57(5):2014–2025. doi: 10.1002/hep.26197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lepiller Q, Abbas W, Kumar A, Tripathy MK, Herbein G. HCMV activates the IL-6-JAK-STAT3 axis in HepG2 cells and primary human hepatocytes. PLoS ONE. 2013;8(3):e59591. doi: 10.1371/journal.pone.0059591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahamid M, Paz K, Reuven M, Safadi R. Hepatotoxicity due to tocilizumab and anakinra in rheumatoid arthritis: two case reports. Int J Gen Med. 2011;4:657. doi: 10.2147/IJGM.S23920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse ME, Lockshin MD, et al. 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res. 2020;72(4):461–488. doi: 10.1002/acr.24130. [DOI] [PubMed] [Google Scholar]
- 45.Paccaly AJ, Kovalenko P, Parrino J, Boyapati A, Xu C, van Hoogstraten H, DiCioccio AT. Pharmacokinetics and pharmacodynamics of subcutaneous sarilumab and intravenous tocilizumab following single-dose administration in patients with active rheumatoid arthritis on stable methotrexate. J Clin Pharmacol. 2021;61(1):90–104. doi: 10.1002/jcph.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Information on COVID-19 patients treated with TCZ or SAR obtained from the FEARS database.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html#collapse2