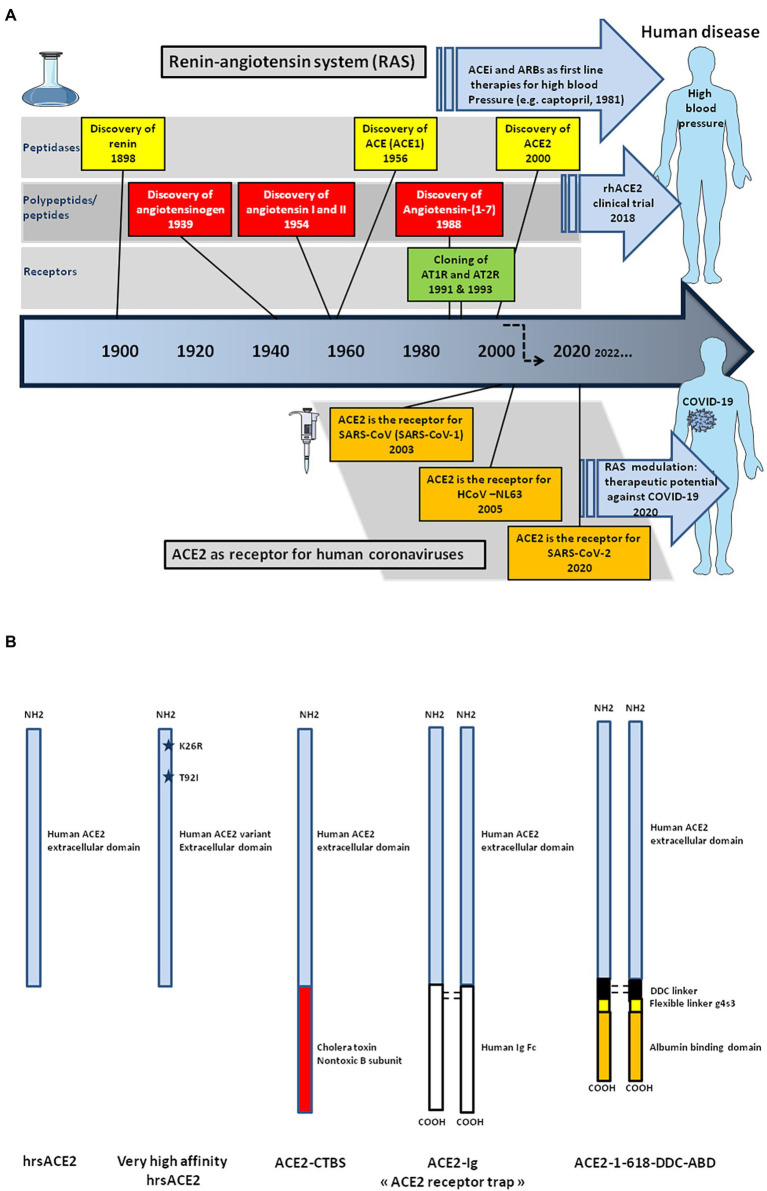
Figure 10.
Schematic representation of the historical discovery of the main components of the renin-angiotensin system (RAS) and ACE2 candidate therapeutic molecules. (A) In 1898, renin was the first component of the RAS to be discovered. Vasoconstriction of the renal artery was then shown to lead to high blood pressure, thus driving the discovery of hypertensin and angiotonin (a compound later termed angiotensin). Angiotensin was subsequently characterized, as well as two downstream conpounds, the Ang I and Ang II, respectively. The ACE peptidase responsible for processing of Ang I into Ang II was subsequently characterized in 1956. The first orally active angiotensin-converting enzyme inhibitor, captopril, was used as antihypertensive therapy in patients with high blood pressure from the early 80s. The AT1R receptor was cloned in 1991, followed by the cloning of AT2R. Then, the counter-regulatory pathway of RAS was described in 2000, with the discovery of ACE2 by two independent research groups and identification of the Ang-(1–7)/Mas receptor interacting partners was achieved two decades later. The cardioprotective effects of ACE2 were discovered as was its ability to process Ang II into Ang-(1–7). Finally, studies have identified the ACE2 protease domain as the receptor for severe acute respiratory syndrome-coronavirus (SARS-CoV-1) in 2003, HCoV-NL63 in 2005, and, more recently (2020), SARS-CoV-2. (B) The ACE2 peptidase extracellular domain known to bind the SARS-CoV-2 Spike can be produced as a recombinant soluble molecule able to neutralize SARS-CoV-2. Various amino acid substitutions can be introduced in the sequence of the ACE2 extracellular domain by genetic engeneering to change the affinity of the recombinant molecule for the viral spike. However, hrsACE2 appears to have a short half-life the efficiency of which could be improved by engineering fusion proteins. The fusion of the rhACE2 extracellular domain with the nontoxic subunit B of cholera toxin (ACE2-CTBS) improves transmucosal transport. The recombinant ACE2-Ig fusion protein consists of a homodimer of the ACE2 extracellular domain linked to an Fc domain of human IgG increasing the stability of the molecule but could also act as cargo for the virus through the Fc-Tag to attach cells like macrophages that express high levels of the Fc receptor. The addition of an albumin binding domain in fusion with ACE2 extend the duration of ACE2 action.