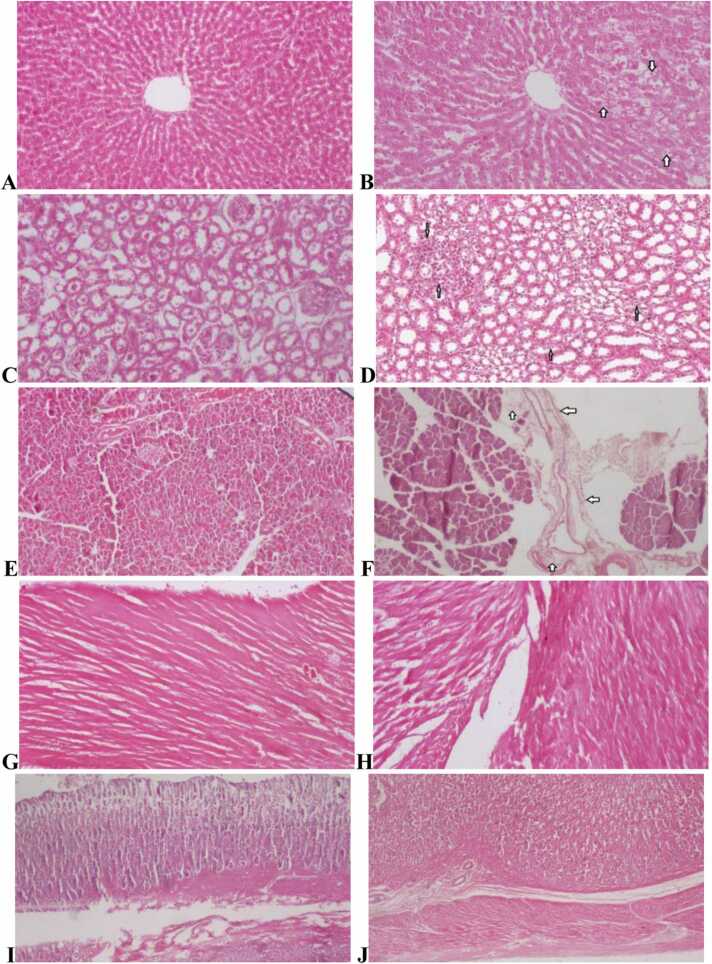
Fig. 3.
Histopathology of the liver, kidneys, heart, panaceas and stomach in subchronic toxicity study (H & E stained). (A) Normal histology of the liver from controls (x10); (B) Mild hydropic changes of the liver at 2237.44 mg/kg (x10); (C) Normal histology of the kidney from control group (x20); (D) Mild-focal inflammation of the kidney at 2237.44 mg/kg group (x20); (E) Normal architecture of the pancreas from control rats (x10); (F) Mild inflammation of the pancreas at 2237.44 mg/kg group (x20); (G) Normal cardiac myocytes appearance from control group (x10); (H) Normal cardiac architecture at 2237.44 mg/kg group (x20); (I) Normal histology appearances of the stomach from control rat (x20); and (J) Normal microscopic appearances of the stomach at 2237.44 mg/kg group (x20).