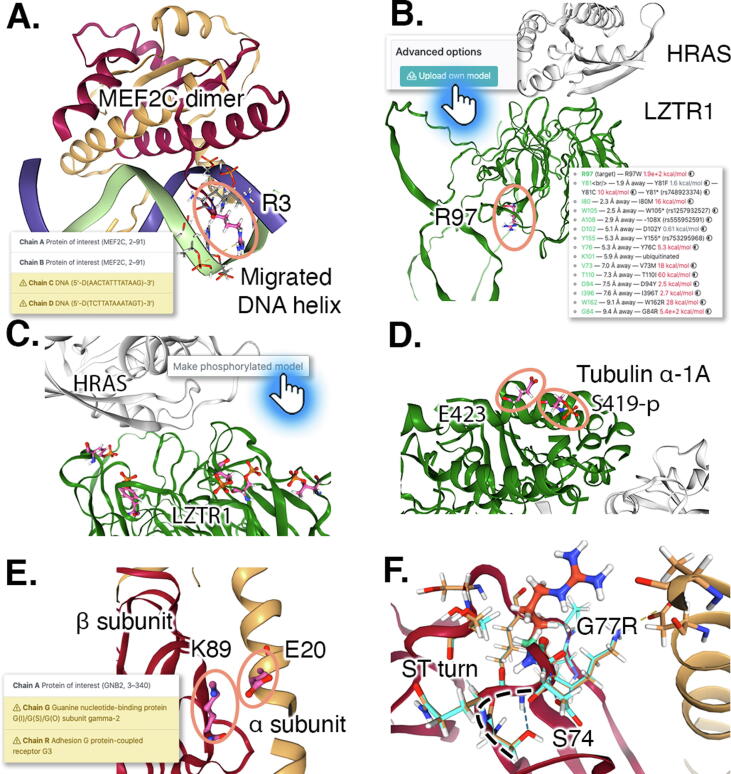
Figure 2.
Examples of variant impact analysis with Venus, illustrating user-focused features and residues investigated circled. (Panel A) The effect of certain variants may be best interpreted in the context of a protein’s known binding partners: portraying MEF2C as a homodimer with the DNA copied from the MEF2A template reveals that the R3C substitution affects DNA binding and nucleotide specificity. In the structural information element of the left-hand side card of Venus the chains are listed and the copied chains flagged for further consideration (inset). (Panel B) The models available may not always be ideal and in certain cases providing Venus with a custom model is important to investigate a variant, as illustrated by the LZTR1:HRAS complex. Furthermore, the presence and effect on stability of nearby gnomAD variants may help formulate a hypothesis. In the case of LZTR1 R97L, these reveal that it is not an interface residue and that most gnomAD variants are highly destabilising, including R97W, in contrast to R97L, which is near neutral. (Panel C–D) Several variants are adjacent to phosphorylated residues, therefore it is important to have the option to make a model of these, as seen for the LZTR1 interface and Tubulin α-1A E423G, which is close to S419, a target of phosphorylation. (Panel E) In Venus, emphasis is placed on user inspection and interaction, as opposed to giving a single metric. The potential effect of certain variants may be multifaceted, for example in G-protein β2, subunit K89 forms a salt bridge with E20 of the α subunit (migrated chains in insert), but the substitution to threonine has a compensating stabilising effect, resulting in an overall neutral ΔΔG, furthermore, the residue is a ubiquitination target. (Panel F) The inspection of the overlay of models for wild type (teal/turquoise) and variant G77R (coral/gold) of the G-protein β2 subunit allows the formulation of the hypothesis that the G77R substitution in G-protein β2 subunit may affect the conformation adopted by the phosphorylation of S74, even if this is not available.