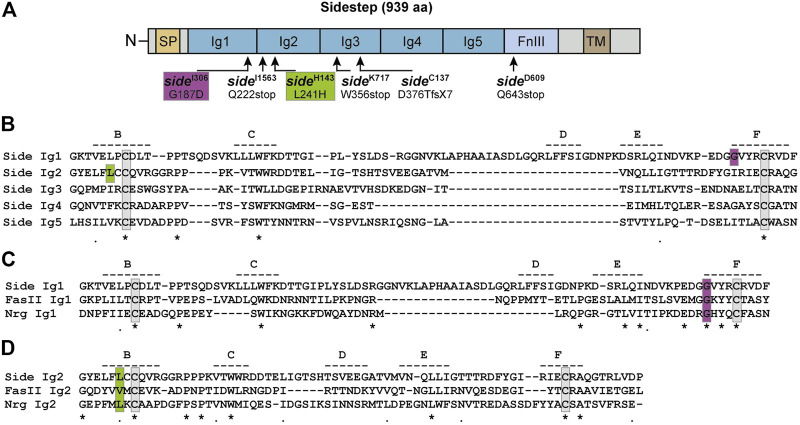
FIGURE 1.
Single point mutations in side disrupt conserved amino acids. (A) Domain structure of Sidestep (Side). Arrows indicate positions of mutagen-induced point mutations. Alleles and their amino acid changes are indicated. aa, amino acids; fsX7, frame shift inducing stop codon after seven aa; SP, signal peptide; Ig, immunoglobulin domain; FnIII, fibronectin type-III domain; TM, transmembrane domain. Not to scale. (B) Sequence alignment of the five Ig domains of Side. The approximate positions of potential β-strands are indicated by dashed lines and labelled B through F. Strands A and G are not depicted. The D strand is misaligned due to a sequence insertion in Ig1. Identical and similar amino acids are marked by asterisks and periods, respectively. Essential cysteines are boxed (grey). The mutated glycine in side I306 and leucine in side H143 are boxed magenta and green, respectively. (C) Alignment of the first Ig domains of the structurally, but not functionally, related proteins Side, Fasciclin II (FasII) and Neuroglian (Nrg). Essential cysteines are boxed (grey). The mutated glycine in side I306 is highly conserved (boxed, magenta).(D) Alignment of the second Ig domains of Side, FasII and Nrg. Hydrophobic amino acids are conserved at and next to the mutated leucine (boxed, green).