FIGURE 7.
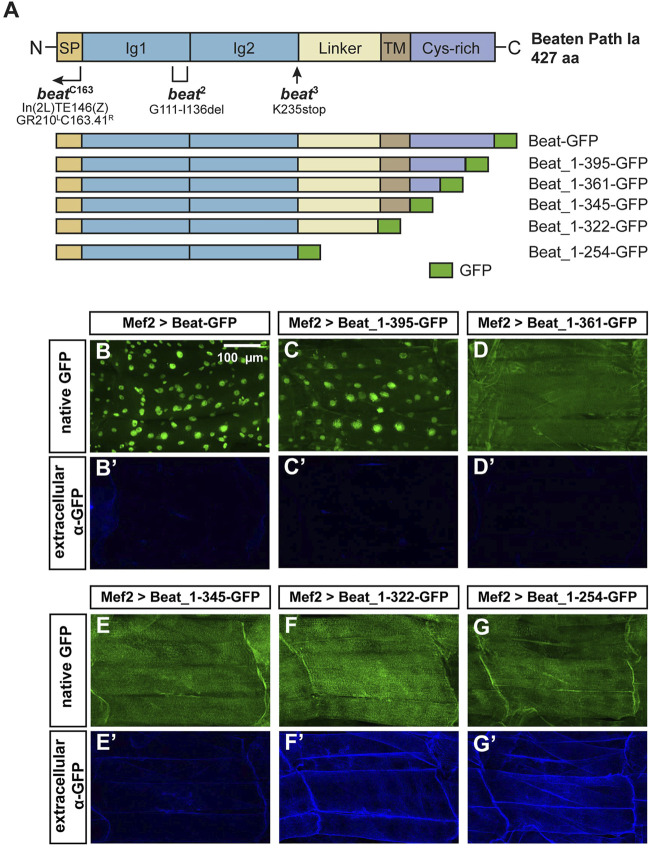
N-terminal domains of Beat are exposed on muscle surfaces.(A) Upper panel: Domain structure of Beat (427 aa). Bracket shows the extent of the deletion in beat 2. Arrow indicates position of the point mutation in beat 3. Right-angled arrow marks the approximate breakpoint of the inversion in beat C163 (Fambrough and Goodman, 1996). SP, signal peptide; Ig, immunoglobulin domain; Linker, sequence enriched in glycine and serine residues. TM, potential transmembrane domain; Cys-rich, cysteine-rich domain. Not to scale. Lower panel: Schemes of Beat C-terminal deletions used in this study. Amino acids of Beat are indicated by numbers, GFP was inserted at the C-terminus. (B–G') Confocal images of non-permeabilized fillet preparations of third instar larvae expressing the indicated Beat constructs in muscles under control of Mef2-Gal4. (B–G) Native GFP fluorescence of the fusion proteins. (B'–G') Extracellular GFP as detected by anti-GFP antibodies. (B–C') GFP tags of Beat-GFP and Beat_1–395-GFP accumulate in nuclei but not on muscle surfaces. (D–E') Beat_1–361-GFP and Beat_1–345-GFP distribute homogenously but GFP remains intracellularly. (F–G') Beat_1–322-GFP and Beat_1–254-GFP lacking the potential transmembrane domain and the Cys-rich region expose GFP extracellularly.