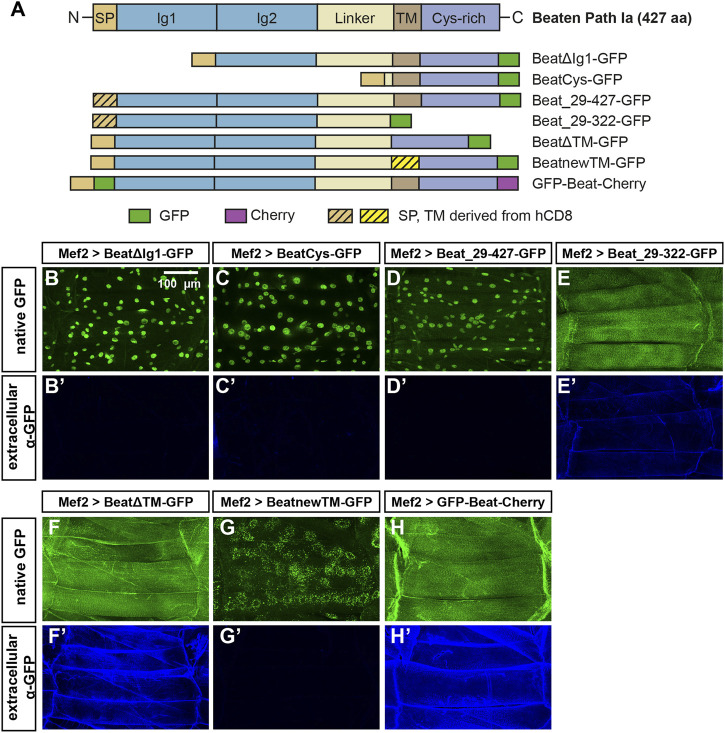
FIGURE 8.
The potential transmembrane domain and the Cys-rich domain are sufficient for nuclear import. (A) Domain structure of wild-type Beat (427 aa) and Beat constructs used in this study. Encoded amino acids of Beat and the positions of fluorescent tags (GFP, green; Cherry, magenta) are indicated. Sequences derived from human CD8 are hatched. Other abbreviations as in Figure 7. Not to scale. (B–H') Confocal images of non-permeabilized fillet preparations of third instar larvae expressing the indicated Beat constructs in muscles under control of Mef2-Gal4. (B–H) Native GFP fluorescence. (B'–H') Extracellular GFP as detected by anti-GFP antibodies. (B–C') Deletion of the first or the first two Ig domains causes C-terminal GFP to accumulate in muscle nuclei but not on muscle surfaces. (D–E') Replacement of the endogenous signal peptide by a generic version from human CD8 does neither affect nuclear localization (Beat_29–427-GFP) nor extracellular exposure of GFP (Beat_29–322-GFP). (F–G') Deletion of the transmembrane region in BeatΔTM-GFP leads to exposure of GFP on muscle surfaces (F–F). Exchange of the transmembrane domain traps BeatnewTM-GFP in intracellular, perinuclear ER compartments (G). (H–H') GFP fused to the N-terminus of Beat distributes homogenously on muscle surfaces.Scale bar: 100 μm.