Abstract
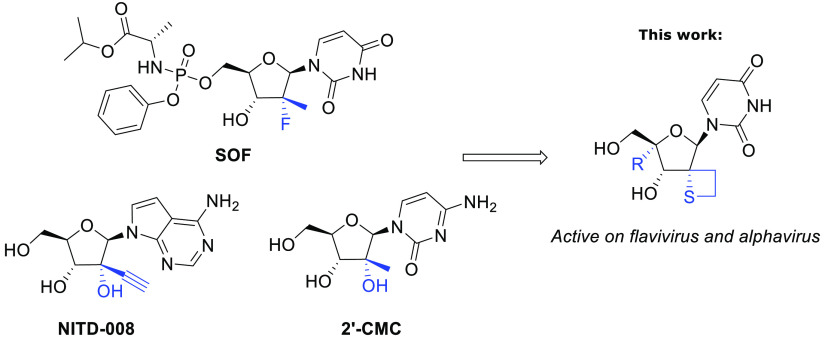
In continuation of our efforts of finding novel nucleoside inhibitors for the treatment of viral diseases, we initiated a discovery research program aimed at identifying novel nucleos(t)ide inhibitors for emerging diseases like Dengue and Chikungunya. Based on the previously reported 2′-spiro-oxetane uridine derivatives active against Hepatitis C Virus (HCV), we envisaged its sulfur analogue as an interesting congener both from a synthetic as well as biological point of view. Surprisingly, we found the 2′-spirothietane uridine derivatives not only to be active against HCV and Dengue virus (DENV), viruses belonging to the flavivirus family, but also to demonstrate activity against alphaviruses like Chikungunya virus (CHIKV) and Sindbis virus (SINV).
Keywords: Nucleos(t)ide, thietane, spirocycle, Hepatitis C, Dengue, Chikungunya
Synthetic nucleosides and nucleotide analogues represent an important class of antiviral, antiparasitic, and anticancer therapeutics that have been widely applied in clinical practice.1 For example, Sofosbuvir, a 4′-modified nucleoside, often used in combination with other antivirals, has played a key role in the fight against Hepatitis C (HCV).2−4 Lately nucleosides derivatives have regained great interest from the scientific community with the recent approval of Remdesivir and Molnupiravir for SARS COV-2 treatment.5−7 While the current focus on finding treatment options against COVID-19 is understandable, there are other pathogens of concern for which no effective therapies exist. Infections caused by dengue virus (DENV) or chikungunya virus (CHIKV) are expected to spread on a global scale,8 and reliable treatment options for patients suffering from these diseases are needed. NITD-008, an adenine derivative bearing a 2′-ethynyl fragment, has demonstrated antiviral activity against DENV in cell based assays9 as did 2′-C-methyl cytidine (2′-CMC).10
To exert any antiviral activity, most nucleoside analogues need to be converted into the bioactive nucleoside triphosphate (NTP) metabolite which acts in a competitive manner to natural NTPs that are used by viral polymerases to synthesize de novo RNA or DNA. Incorporation of these unnatural nucleosides results in chain elongation termination which prevents the formation of newly generated viral particles.11
Structural modification of the ribose moiety of nucleosides is a well-documented approach that has been demonstrated to be a common tool to modulate biological properties of nucleoside analogues.12−14 Such strategies even led to approved antiviral drugs.15
It has been shown that ribose modifications can include spirocyclization at various positions16−18 which is confirmatory for the increasing prominence of spirocyclic scaffolds in drug discovery programs.19,20 The use of small rings like cyclopropyl, oxetane, or azetidine provides access to denser, more rigid molecular structures which often confer beneficial properties compared to their nonspirocyclic analogues.
Thietane, the sulfur analogue of the well-known oxetane is an important structural motif found in natural products and pharmacologically active ingredients.21 A 4′-5′ spirothietane modified sugar analogue was disclosed by Roy et al.22,23
Aiming to identify novel antiviral active nucleoside analogues and extending on our previous work24−28 we investigated further modifications of the 2′-region of uridine with original spiro-thietane motifs (Figure 1).29 To the best of our knowledge, no 2′-spirothietane nucleosides have been reported so far.
Figure 1.
2′-Spirothietane ribose derivatives active on flavivirus and alphavirus.
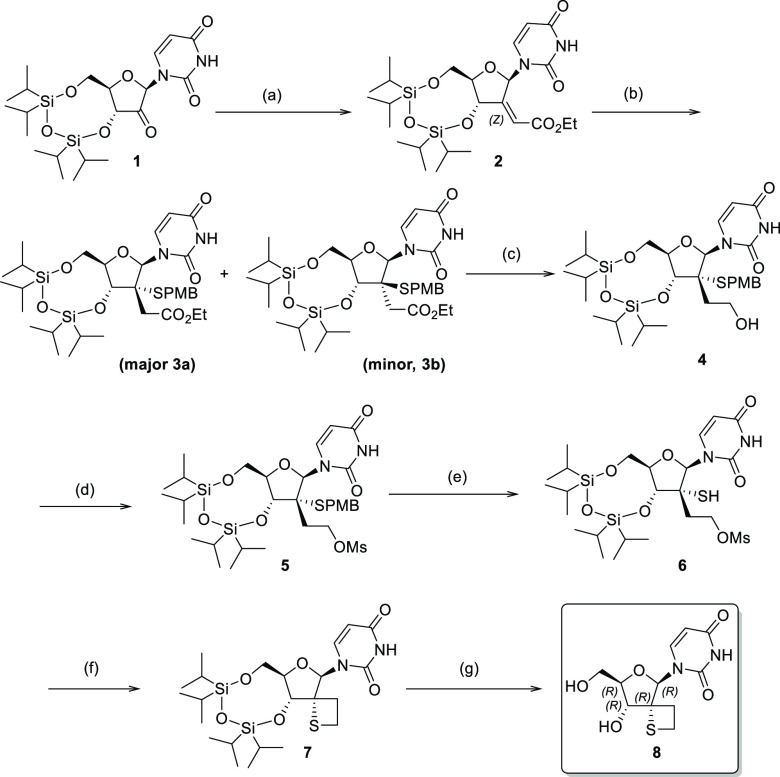
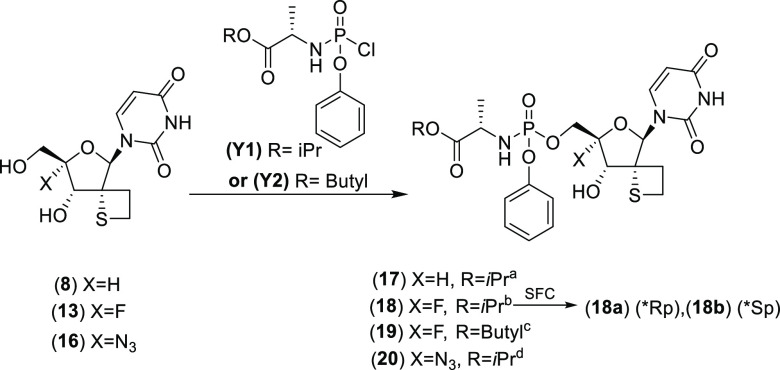
Our synthetic approach toward 2′-spirothietane uridine derivatives started from the previously reported ketone 1 (Scheme 1).24 The key step in the sequence was the Michael reaction of the α,ß-unsaturated ester 2 with (4-methoxyphenyl)methanethiol leading to intermediates 3a and 3b with the desired S-α-isomer being formed predominantly. Subsequently, compound 5 was obtained in good yield after reduction of 3a followed by mesylation of the primary alcohol. Mercury mediated deprotection of the para methoxybenzyl group, followed by intramolecular cyclization in basic conditions afforded compound 7 in a moderate yield of 46% over 2 steps. Finally, deprotection of the hydroxyl groups led to the novel 2-spirothietane nucleoside 8, a key intermediate.
Scheme 1. Synthesis Access to Spirothietane Nucleoside 8.
Conditions: (a) (EtO)2P(O)CH2CO2Et, tBuOK, THF, −15 °C to RT (1 h), then 1 in THF 0 °C to 15 °C (1 h) then RT (5 h) 83%. (b) (4-methoxyphenyl)Methanethiol, KHMDS; THF, −40 to 20 °C (2 h), 50% (3a) 12% (3b). (c) LiAlH4, Et2O, 0 to 20 °C (16 h) 60%. (d) MsCl, pyridine, 25 °C (16 h), 78%. (e) Hg(OAc)2, CF3CO2H, PhOH, 0 °C (1 h), then DTT, 0 °C (10 min). (f) NaH, THF, 0 to 25 °C (16 h), 46% over 2 steps. (g) TBAF, THF, RT (2 h), 86%.
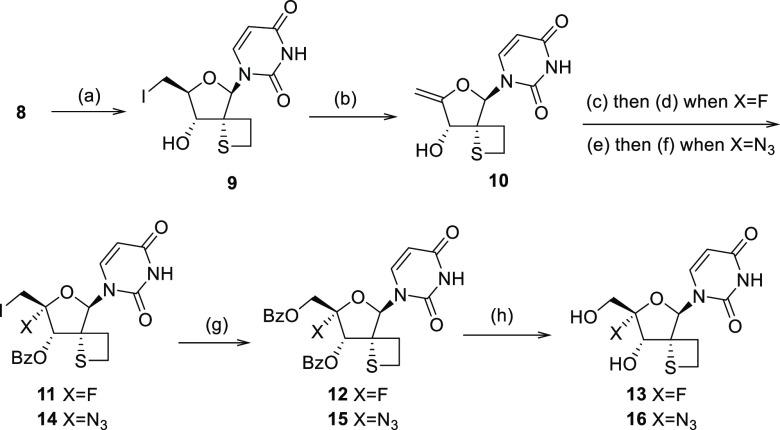
This synthetic protocol allowed us to obtain multigram quantities of 8 and set us up to further extend our exploration of this new 2′-spirothiethane scaffold. The synthetic approach to install a 4′-F substituent was based on initial work reported by Moffat et al.30 which was also extensively exploited by others31−34 (Scheme 2). First, compound 8 was reacted with iodine in the presence of triphenylphosphine followed by an elimination step leading to 4′,5′-unsaturated nucleoside 10. Iodofluorination of compound 10 using N -iodosuccinimide (NIS) and Et3N(HF)3, yielded 4′-fluoro-5′-iodo derivative 11 in 62% yield. After benzoylation of the 3′-hydroxy group, displacement of the iodine by means of sodium benzoate afforded the double protected nucleoside 12 which after treatment with ammonia led to the fully deprotected 4′-F, 2′-spirothietane nucleoside 13 in good yield. The 4′-azido nucleoside 16 was obtained in a similar manner utilizing sodium azide in step 3. Nucleoside prodrug approaches like “Protides/phosphoramidates” are well-documented. Such technologies are aimed at circumventing any problematic first phosphorylation which is often observed with unnatural derivatives.35 5′-Phosphoramidate analogues 17–20 were obtained using standard conditions as mixtures of P-stereoisomers30 (Scheme 3). While stereoselective synthetic methods are reported, we opted for a nonstereoselective synthesis for initial evaluation. In the case of 18, preparative SFC (Supercritical Fluid Chromatography) yielded the enantiopure phosphoramidates 18a (*Rp) and 18b (*Sp) of which the stereochemistry at the P-atom was assigned arbitrarily.
Scheme 2. Synthetic Access to 4′-Functionalized Spirothietane Nucleosides.
Conditions: (a) I2, P(Ph)3, NMI, THF, 25 °C, 4 h. (b) MeONa, MeOH, reflux, 2 h, 55% over 2 steps. (c) Et3N(HF)3, THF/ACN, NIS, −15 °C (1 h). (d) Et3N, DMAP, THF, BzCl, 0 to 25 °C (3 h), 62%. (e) (1) BnEt3NCl, NaN3, CH3CN, RT (16 h) then 10, NMM, I2, THF, 0 °C to RT (5 h), 99%. (f) BzCl, Et3N, DMAP, THF, RT (2 h), 81%. (g) BzONa, 15-crown-5, DMF, 120 °C (18 h), 59% when X = F, 47% when X = N3. (h) NH3, MeOH, RT, overnight, 65% when X = F, 84% when X = N3.
Scheme 3. 5′-Phosphoramidate Nucleosides Synthesis.
Conditions: (a) Y1, DCM, NMI, 20°C (16 h), 12%. (b) tBuMgCl, THF, −5°C (45 min.) then Y1, −5°C (2 h) then RT (overnight), 38%. (c) Y2, NMI, DCM, (overnight), 17%. (d) Y1, NMI, DCM, RT (20 h), 22%.
Cell-based antiviral activity of phosphoramidates 17–20 was evaluated (Table 1). The non-4′-substituted thietane analogue 17 demonstrated broad antiviral activity against CHIKV, HCV, and DENV without apparent cytotoxicity up to 100 μM. Interestingly, the corresponding 4′-fluoro analogue 18 showed a similar extended antiviral profile, with a marked improvement on CHIKV inhibition. In contrast, the 4′-azido derivative 20 displayed reduced potency against DENV and CHIKV. No significant difference in activity was noted between the pure diastereomers 18a and 18b. Phosphoramidate 19, having a butyl chain replacing the terminal isopropyl group in 18, demonstrated similar potencies. Interestingly, phosphoramidates 18, 18a, 18b, and 19 also demonstrated antiviral activity against Sindbis virus (SINV), another virus from the alphaviruses family. This illustrates again the extended antiviral profile of the 2′-spirothietane 4′-F-modified nucleosides. Noteworthy is the fact that 17 and 18 having a sulfur-ring surprisingly show improved activity when compared with their corresponding oxetane analogues.13
Table 1. Antiviral Activity against CHIKV, SINV, HCV, and DENV, and Cytotoxicity in Huh-7 cells for 2′-Thietane Analogues and the 2′-Oxetane Reference Nucleosidesa.
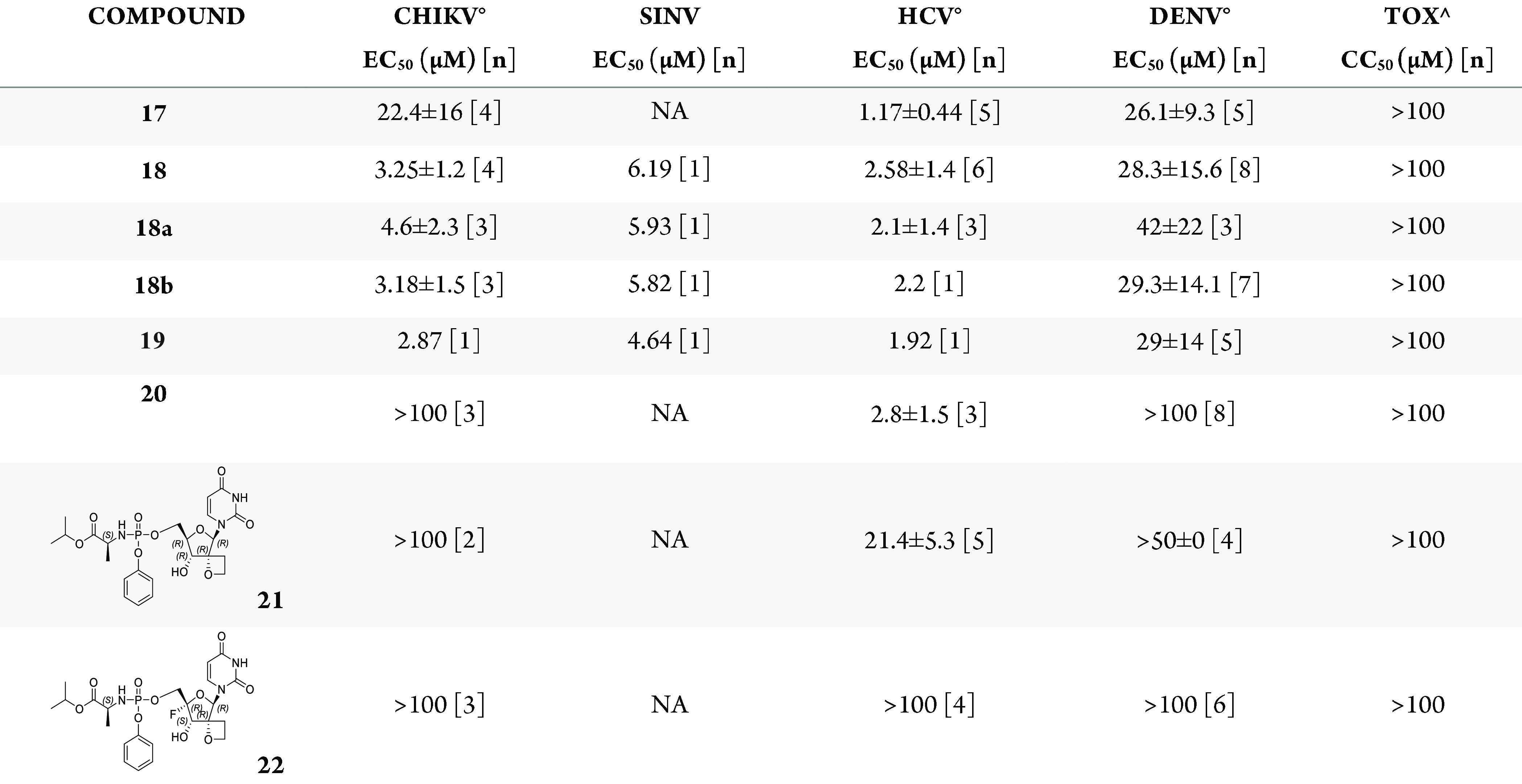
Data represent mean values of [n] independent experiments. °Mean values generated with two different compound dispensing methods. ∧Identical results were obtained in two different toxicity assays (2-day and 3-day assay) in Huh-7 cells. CC50, 50% cytotoxic concentration; EC50, 50% effective concentration. Reference compounds behaved as expected in the different antiviral assays.
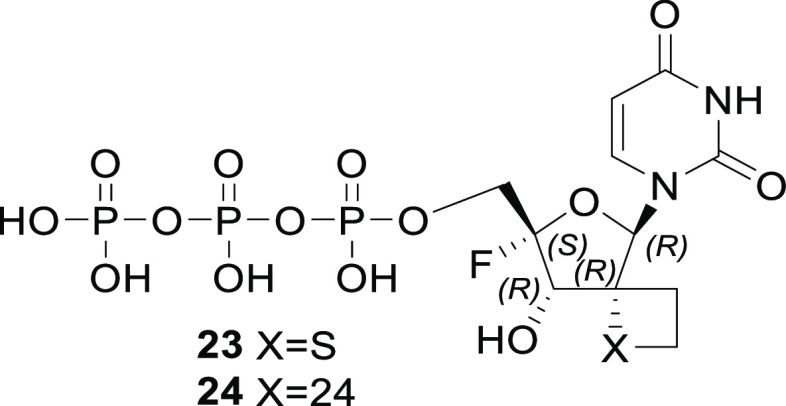
In order to rationalize the broad spectrum antiviral activity of the compounds, we modeled the binding interaction of the thietane-2′-spirocyclic uridine triphosphate 23 and the corresponding oxetane compound 24 (Figure 2) into the crystal structure available for the HCV NS5B and compared the primary sequences for DENV NS5, CHIKV NSP4 and SINV NSP4 for the ligand binding domain and especially the thietane and oxetane microenvironment (Figure 3).
Figure 2.
Structure of triphosphates 23 and 24.
Figure 3.
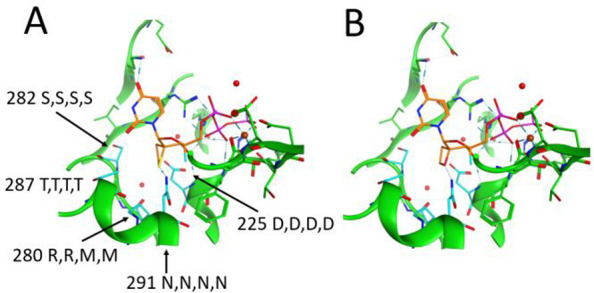
Model of the thietane-2′-spirocyclic uridine triphosphate compound 23 (Panel A) and corresponding oxetane compound 24 (Panel B) into the crystal structure available for the HCV NS5B (PDB 4WTG). Selected residues within a 5 Å contact sphere of the ligand are shown. Carbon atoms of the ligand are colored in orange. Highlighted in cyan are the contact residues of the thietane/oxetane rings. These residues are numbered according to the HCV NS5B sequence, and sequence conservation is indicated: the first letter is the amino acid in HCV NS5B, the second letter is the amino acid in DENV NS5, the third letter is the amino acid in CHIKV NSP4, and the fourth letter is the amino acid in SINV NSP4.
The modeling was based on the HCV NS5B crystal structure in complex with sofosbuvir diphosphate and a symmetrical RNA primer template.36 The thietane and oxetane-2′-spirocyclic system was built in MOE2020.0101 software based on the sofosbuvir 2′-fluoro-2′-methyl ribose coordinates (PDB 4WTG). The γ-phosphate group was modeled based on the coordinates of the Norwalk virus crystal structure complex with a cytidine triphosphate (PDB 3BSO).36,37 The canonical RNA-dependent RNA polymerase architecture resembles a right-hand with palm, fingers and thumb domains surrounding the active site. Polymerase catalytic motifs A-E in the palm and motifs F-G in the fingers are shared by all viral RNA-dependent RNA polymerase with sequence and/or structural conservations.38 The analysis of the putative interactions shows that ASN291 (Motif B) is a key contact residue with a H-bond to the sulfur atom of the thietane ring (acceptor donor distance 3.147 Å).39
The hydrophobic thietane ring is surrounded by the nonpolar carbon atoms of the side chains of ASP225 (Motif A) and ARG280, SER282, and THR287 (all Motif B). The analysis of the sequence conservation shows that the nature of these residues and their surroundings are highly conserved also in DENV NS5, CHIKV NSP4 and SINV NSP4 which might thus provide a rationale for the conservation of binding. The inactivity or lower potency of the oxetanes compared to the thietanes can possibly be due to the stronger interaction of the side chain N–H of ASN 291 to the sulfur atom of the thietane ring.39 The acceptor–donor distance is in the oxetane complex, 3.324 Å, and thus is longer than that in the thietane complex. The C3 OH group interacts via H-bonds with the N–H group of ASP225 and the β-phosphate group. The C4 fluorine atom points toward the side chain NH2 group of ASN291. These observations provide a potential rationale for the measured antiviral activity. However, in the absence of any crystal structure, this remains hypothetical.
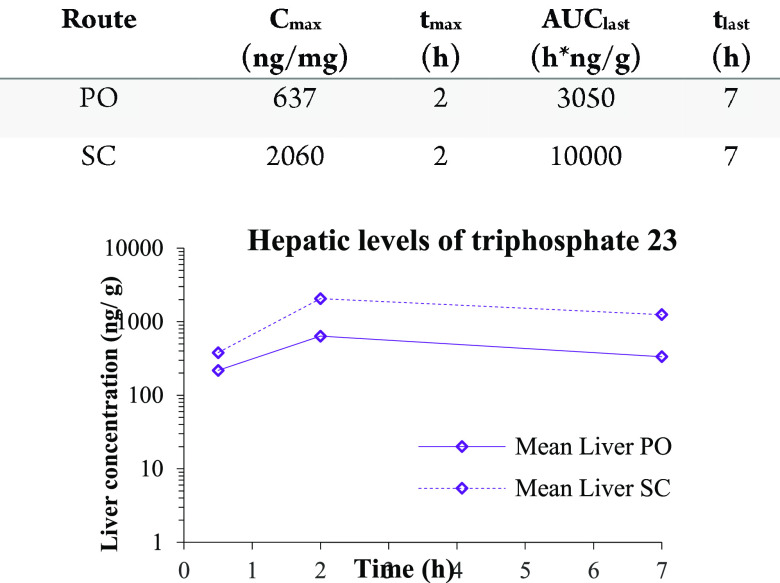
Phosphoramidate 18 was evaluated in a mouse PK experiment to demonstrate the formation of the corresponding NTP 23 upon subcutaneous and/or oral administration. As can be seen from the Figure 4, efficient formation of NTP 23 was shown in the liver. No prodrug was observed in the plasma or the liver after 7 h. The highest liver concentrations of NTP were obtained after subcutaneous dosing of compound 18.
Figure 4.
PK profile in mouse liver after subcutaneous or oral administration of phosphoramidate 18. Samples of plasma and liver were collected. Liver was analyzed for the prodrug 18 and triphosphate 23. Plasma samples were only analyzed for prodrug. [Dose 50 mg/kg, Dose Volume 10 mL/kg, Fed Mice Male, Vehicle HP-ß-CD 20% (solution]). LLOQ [PO] = 90.1–126 ng/g, LLOQ [SC] = 91.8–124] ng/g.
To further increase our understanding of these results, the metabolic stability in mouse and human liver microsomes as well as in vitro stability of compound 18 in simulated gastric fluid (SGF), fasted state simulated intestinal fluid (fassif) and plasma (human and mouse) were evaluated (Tables 2 and 3). Phosphoramidate 18 demonstrated poor metabolic stability both in mouse and human liver microsomes, indicating a fast liver mediated metabolism. While being highly stable in SGF (t1/2 > 6 h, 93 ± 5% of compound remaining after 6 h) and human plasma, compound 18 also showed high instability in fassif and mouse plasma. Results suggest fast turnover of the prodrug in plasma which is not surprising knowing that phosphoramidate esters are prone to extensive metabolization in rodent plasma given the high abundance of carboxylesterases in this medium.40 The combination of the instabilities and limited oral absorption potentially explains differences between oral and subcutaneous dosing. Complementary studies would be required to identify if additional metabolic pathways are involved.
Table 2. Stability of Phosphoramidate 18 in Different Media.
| medium | t1/2 (h) |
|---|---|
| fassif | 0.2 |
| SGF | >6 |
| mouse plasma | <0.4 |
| human plasma | >30 |
Table 3. Metabolic Stability of Phosphoramidate 18 in Mouse and Human Liver Microsomes.
| Clint (μL/min/mg protein) | |
|---|---|
| mouse | >347 |
| human | >347 |
Several novel 2′-spirothietane uridine analogues were found to be inhibitors of pathogens belonging to different viral families (flavivirus and alphavirus). Preliminary docking is in line with the observed activities. Finally, initial PK confirmed the formation of active triphosphate in the liver.
Acknowledgments
The authors thank Guillaume Brunet and Lili Hu for their synthetic contributions and members of the purification and analysis team from Beerse, the biology teams including Edwin Gong, and Guenter Kraus and ADMET team from Janssen R & D.
Glossary
Abbreviations
- HCV
Hepatitis C Virus
- DENV
Dengue Virus
- CHIKV
Chikungunya Virus
- SINV
Sindbis Virus
- NTPs
nucleoside triphosphates
- NIS
N-iodosuccinimide
- SFC
supercritical fluid chromatography
- RT
room temperature
- PK
pharmacokinetics
- NA
not available
- LLOQ
lower limit of quantification
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00372.
Author Present Address
† Galapagos, General De Wittelaan L11, A3, 2800 Mechelen, Belgium
Author Present Address
‡ Galapagos, 102 Avenue Gaston Roussel, 93230 Romainville, France
Author Present Address
§ Van Eycklei 15, 2018, Antwerp, Belgium
Author Contributions
∥ S.G. and A.T. contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Li G.; Yue T.; Zhang P.; Gu W.; Gao L.-J.; Tan L. Drug Discovery of Nucleos(t)ide Antiviral Agents: Dedicated to Prof. Dr. Erik De Clercq on Occasion of His 80th Birthday. Molecules. 2021, 26, 923–938. 10.3390/molecules26040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia M. J.; Bao D.; Chang W.; Du J.; Nagarathnam D.; Rachakonda S.; Reddy P. G.; Ross B. S.; Wang P.; Zhang H.-R.; Bansal S.; Espiritu C.; Keilman M.; Lam A. M.; Micolochick Steuer H. M.; Niu C.; Otto M. J.; Furman P. A. Discovery of a β-d-2′-Deoxy-2′-α-fluoro-2′-β-C-methyluridine Nucleotide Prodrug (PSI-7977) for the Treatment of Hepatitis C Virus. J. Med. Chem. 2010, 53, 7202–7218. 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]
- EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020, 71, 686–721. 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.veklury.com, consulted 2022/09/26.
- https://www.who.int/news/item/03-03-2022-molnupiravir, consulted 2022/09/26.
- Fischer W.; Eron J. J.; Holman W.; Cohen M. S.; Fang L.; Szewczyk L. J.; Sheahan T. P.; Baric R.; Mollan K. R.; Wolfe C. R.; Duke E. R.; Azizad M.; Borroto-Esoda K.; Wohl D. A.; Loftis A. J.; Alabanza P.; Lipansky F.; Painter W. P. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci. Transl. Med. 2022, 14, 1–10. 10.1126/scitranslmed.abl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S. C.; Charlier C.; Vasilakis N.; Lecuit M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu. Rev. Med. 2018, 69, 395–408. 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z.; Chen Y.-L.; Schul W.; Wang Q.-Y.; Gu F.; Duraiswamy J.; Kondreddi R. R.; Niyomrattanakit P.; Lakshminarayana S. B.; Goh A.; Xu H. Y.; Liu W.; Liu B.; Lim J. Y. H.; Ng C. Y.; Qing M.; Lim C. C.; Yip A.; Wang G.; Chan W. L.; Tan H. P.; Lin K.; Zhang B.; Zou G.; Bernard K. A.; Garrett C.; Beltz K.; Dong M.; Weaver M.; He H.; Pichota A.; Dartois V.; Keller T. H.; Shi P.-Y. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 20435–20439. 10.1073/pnas.0907010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-C.; Tseng C.-K.; Wu Y.-H.; Kaushik-Basu N.; Lin C.-K.; Chen W.-C.; Wu H.-N. Characterization of the activity of 2’-C-methylcytidine against dengue virus replication. Antiviral Res. 2015, 116, 1–9. 10.1016/j.antiviral.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Mishra S.; Shreya; Maurya S. K. Recent advances in the discovery of potent RNA-dependent RNA-polymerase (RdRp) inhibitors targeting viruses. RSC Med. Chem. 2021, 12, 306–320. 10.1039/D0MD00318B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seley-Radtke K. L.; Thames J. E.; Waters C. D. Chapter Three - Broad spectrum antiviral nucleosides - Our best hope for the future. Annu. Rep. Med. Chem. 2021, 57, 109–132. 10.1016/bs.armc.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ami E.-I.; Ohrui H. Intriguing Antiviral Modified Nucleosides: A Retrospective View into the Future Treatment of COVID-19. ACS Med. Chem. Lett. 2021, 12, 510–517. 10.1021/acsmedchemlett.1c00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdewijn P.Modified Nucleosides: in Biochemistry, Biotechnology and Medicine; Wiley-VCH Verlag GmbH & Co. KGaA, 2008. [Google Scholar]
- Chang J. 4′-Modified Nucleosides for Antiviral Drug Discovery: Achievements and Perspectives. Chem. Res. 2022, 55, 565–578. 10.1021/acs.accounts.1c00697. [DOI] [PubMed] [Google Scholar]
- Du J.; Chun B.-K.; Mosley R. T.; Bansal S.; Bao H.; Espiritu C.; Lam A. M.; Murakami E.; Niu; Congrong M.; Steuer H. M.; Furman P. A.; Sofia M. J. Use of 2’-Spirocyclic Ethers in HCV Nucleoside Design. J. Med. Chem. 2014, 57, 1826–1835. 10.1021/jm401224y. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Kumar M.; Kumar V.; Kumar A.; Haque N.; Kumar R.; Prasad A. K. Recent progress in the synthesis of C-4′- spironucleosides and its future perspectives. Synth. Commun. 2020, 50, 3369–3396. 10.1080/00397911.2020.1803914. [DOI] [Google Scholar]
- Neouchy A.; Verhoeven J.; Kong H.; Zhao Y.; Wang W.; Brambilla M.; Van Hecke K.; Meerpoel L.; Thuring J. W.; Verniest G.; Winne J. Stereodivergent synthesis of biologically active spironucleoside scaffolds via catalytic cyclopropanation of 4-exo-methylene furanosides. J. Org. Chem. 2021, 86, 17344–17361. 10.1021/acs.joc.1c01611. [DOI] [PubMed] [Google Scholar]
- Carreira E. M.; Fessard T. C. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114 (16), 8257–8322. 10.1021/cr500127b. [DOI] [PubMed] [Google Scholar]
- Hiesinger K.; Dar’in D.; Proschak E.; Krasavin M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021, 64 (1), 150–183. 10.1021/acs.jmedchem.0c01473. [DOI] [PubMed] [Google Scholar]
- Xu J. Synthesis of Thietanes from Saturated Three-membered Heterocycles. Asian J. Org. Chem. 2020, 16, 1357–1410. 10.3762/bjoc.16.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.; Achari B.; Mandal S. B. An easy access to spiroannulated glyco-oxetane, -thietane and -azetane rings: synthesis of spironucleosides. Tetrahedron Lett. 2006, 47, 3875–3879. 10.1016/j.tetlet.2006.03.175. [DOI] [Google Scholar]
- Maity J. K.; Ghosh R.; Drew M. G. B.; Achari B.; Mandal S. B. Introduction of Vinyl and Hydroxymethyl Functionalities at C-4 of Glucose-Derived Substrates: Synthesis of Spirocyclic, Bicyclic, and Tricyclic Nucleosides. J. Org. Chem. 2008, 73, 4305–4308. 10.1021/jo8002826. [DOI] [PubMed] [Google Scholar]
- Jonckers T. H. M.; Lin T.-I; Buyck C.; Lachau-Durand S.; Vandyck K.; Van Hoof S.; Vandekerckhove L. A. M.; Hu L.; Berke J. M.; Vijgen L.; Dillen L. L. A.; Cummings M. D.; De Kock H.; Nilsson M.; Sund C.; Rydegård C.; Samuelsson B.; Rosenquist Å.; Fanning G.; Van Emelen K.; Simmen K.; Raboisson P. 2′-Deoxy-2′-spirocyclopropylcytidine Revisited: A New and Selective Inhibitor of the Hepatitis C Virus NS5B Polymerase. J. Med. Chem. 2010, 53, 8150–8160. 10.1021/jm101050a. [DOI] [PubMed] [Google Scholar]
- Lemaire S.; Houpis I.; Wechselberger R.; Langens J.; Vermeulen W. A. A.; Smets N.; Nettekoven U.; Wang Y.; Xiao T.; Qu H.; Liu R.; Jonckers T. H. M.; Raboisson P.; Vandyck K.; Nilsson K. M.; Farina F. Practical Synthesis of (2′R)-2′-Deoxy-2′-C-methyluridine by Highly Diastereoselective Homogeneous Hydrogenation. J. Org. Chem. 2011, 76, 297–300. 10.1021/jo101822j. [DOI] [PubMed] [Google Scholar]
- Nilsson M.; Kalayanov G.; Winqvist A.; Pinho P.; Sund C.; Zhou X.-X.; Wähling H.; Belfrage A.-K.; Pelcman M.; Agback T.; Benckestock K.; Wikström K.; Boothee M.; Lindqvist A.; Rydegård K.; Jonckers T. H. M.; Vandyck K.; Raboisson P.; Lin T.-I.; Lachau-Durand S.; De Kock H.; Smith D. B.; Martin J. A.; Klumpp K.; Simmen K.; Vrang L.; Terelius Y.; Samuelsson B.; Rosenquist Å.; Johansson N. G. Discovery of 4′-azido-2′-deoxy-2′-C-methyl cytidine and prodrugs thereof: A potent inhibitor of Hepatitis C virus replication. Bioorg. Med. Chem. Lett. 2012, 22, 3265–3268. 10.1016/j.bmcl.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Jonckers T. H. M.; Vandyck K.; Vandekerckhove L.; Hu L.; Tahri A.; Van Hoof S.; Lin T.-I.; Vijgen L.; Berke J. M.; Lachau-Durand S.; Stoops B.; Leclercq L.; Fanning G.; Samuelsson B.; Nilsson M.; Rosenquist A.; Simmen K.; Raboisson P. Nucleotide Prodrugs of 2′-Deoxy-2′-spirooxetane Ribonucleosides as Novel Inhibitors of the HCV NS5B Polymerase. J. Med. Chem. 2014, 57, 1836–1844. 10.1021/jm4015422. [DOI] [PubMed] [Google Scholar]
- Jonckers T. H. M.; Tahri A.; Vijgen L.; Berke J. M.; Lachau-Durand S.; Stoops B.; Snoeys J.; Leclercq L.; Tambuyzer L.; Lin T.-I.; Simmen K.; Raboisson P. Discovery of 1-((2R,4aR,6R,7R,7aR)-2-Isopropoxy-2- oxidodihydro-4H,6H-spiro[furo[3,2-d][1,3,2]dioxaphosphinine-7,2′- oxetan]-6-yl)pyrimidine-2,4(1H,3H)-dione (JNJ-54257099), a 3′-5′- Cyclic Phosphate Ester Prodrug of 2′-Deoxy-2′-Spirooxetane Uridine Triphosphate Useful for HCV Inhibition. J. Med. Chem. 2016, 59, 5790–5798. 10.1021/acs.jmedchem.6b00382. [DOI] [PubMed] [Google Scholar]
- Tahri A.; Bonfanti J. F.; Jonckers T. H. M.; Raboisson P. J.-M. B.. Spirothietane nucleosides. WO2019043177A1, 2019.
- Owen G. R.; Verheyden J. P. H.; Moffatt J. G. 4’-Substituted nucleosides. 3. Synthesis of some 4’-fluorouridine derivatives. J. Org. Chem. 1976, 41, 3010–3017. 10.1021/jo00880a018. [DOI] [PubMed] [Google Scholar]
- Lee S.; Uttamapinant C.; Verdine G. L. A. Concise Synthesis of 4′-Fluoro Nucleosides. Org. Lett. 2007, 9, 5007–5009. 10.1021/ol702222z. [DOI] [PubMed] [Google Scholar]
- Martínez-Montero S.; Deleavey G. F.; Kulkarni A.; Martín-Pintado N.; Lindovska P.; Thomson M.; González C.; Götte M.; Damha M. J. Rigid 2′,4′-Difluororibonucleosides: Synthesis, Conformational Analysis, and Incorporation into Nascent RNA by HCV Polymerase. J. Org. Chem. 2014, 79, 5627–5635. 10.1021/jo500794v. [DOI] [PubMed] [Google Scholar]
- Wang G.; Lim S. P.; Chen Y.-L.; Hunziker J.; Rao R.; Gu F.; Seh C. C.; Ghafar N. A.; Xu H.; Chan K.; Lin X.; Saunders O. L.; Fenaux M.; Zhong W.; Shi P.-Y.; Yokokawa F. Structure-activity relationship of uridine-based nucleosidephosphoramidate prodrugs for inhibition of dengue virus RNA-dependent RNA polymerase. Bio. Org. Med. Chem. Lett. 2018, 28, 2324–2327. 10.1016/j.bmcl.2018.04.069. [DOI] [PubMed] [Google Scholar]
- Wang G.; Dyatkina N.; Prhavc M.; Williams C.; Serebryany V.; Hu Y.; Huang Y.; Wu X.; Chen T.; Huang W.; Rajwanshi V. K.; Deval J.; Fung A.; Jin A.; Stoycheva A.; Shaw K.; Gupta K.; Tam Y.; Jekle A.; Smith D. B.; Beigelman L. Synthesis and Anti-HCV Activity of Sugar-Modified Guanosine Analogues: Discovery of AL-611 as an HCV NS5B Polymerase Inhibitor for the Treatment of Chronic Hepatitis C. J. Med. Chem. 2020, 63, 10380–10395. 10.1021/acs.jmedchem.0c00935. [DOI] [PubMed] [Google Scholar]
- Pradere U.; Garnier-Amblard E.-C.; Coats S. J.; Amblard F.; Schinazi R. F. Synthesis of Nucleoside Phosphate and Phosphonate Prodrugs. Chem. Rev. 2014, 114, 9154–9218. 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby T. C.; Perry J. K.; Murakami E.; Barauskas O.; Feng J.; Cho A.; Fox D. III; Wetmoren D. R.; McGrath M. E.; Ray A. S.; Sofia M. J.; Swaminathan S.; Edwards T. E. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 2015, 347, 771–775. 10.1126/science.1259210. [DOI] [PubMed] [Google Scholar]
- Zamyatkin D. F.; Parra F.; Alonso J. M.; Harki D. A.; Peterson B. R.; Grochulski P.; Ng K. K. Structural Insights into Mechanisms of Catalysis and Inhibition in Norwalk Virus Polymerase. J. Biol. Chem. 2008, 283, 7705–7712. 10.1074/jbc.M709563200. [DOI] [PubMed] [Google Scholar]
- Wu J.; Liu W.; Gong P. A Structural Overview of RNA-Dependent RNA Polymerases from the Flaviviridae Family. Int. J. Mol. Sci. 2015, 16, 12943–12957. 10.3390/ijms160612943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal H. S.; Wategaonkar S. Nature of the N–H···S Hydrogen Bond. J. Phys. Chem. A 2009, 113, 12763–12773. 10.1021/jp907658w. [DOI] [PubMed] [Google Scholar]
- Bahar F. G.; Ohura K.; Ogihara T.; Imai T. Species Difference of Esterase Expression and Hydrolase Activity in Plasma. J. Pharm. Sci. 2012, 101, 3979–3988. 10.1002/jps.23258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.