Abstract
Microplastics (MPs) are perpetual contaminants that are mostly generated by human activity and are deposited in aquatic ecosystem. MPs may react differently in aquatic organisms depending on their size, surface charge, and concentration. The current investigation examined the interactions of polystyrene (PS) microplastics (of varied charges and sizes) with Scenedesmus obliquus, a unicellular phytoplankton. It is observed that 1 µm PS-MPs produced increased oxidative stress than 12 µm PS-MPs as indicated by total reactive oxygen species (ROS), superoxide and hydroxyl radical generation, and lipid peroxidation results. Additionally, decreased photosynthetic effectiveness, membrane integrity and esterase activity were also observed for the lower sized MPs. Antioxidant enzyme activities like superoxide dismutase (SOD) activity and catalase (CAT) activity correlated well with the oxidative stress generation in the cells. The effects by both the sizes of MPs were dose dependent in nature. Given the importance of a rapidly developing scientific literature on the effects of MPs in freshwater organisms, understanding the dynamics of interactions with lower-level organisms becomes very relevant.
Keywords: Concentration, Oxidative stress, Polystyrene Microplastics, Scenedesmus obliquus, Surface charge, Toxicity
Graphical Abstract
Highlights
-
•
Interaction of polystyrene microplastics with freshwater microalgae was assessed.
-
•
Size and surface charge dependent toxic effects were observed.
-
•
Oxidative stress was the major mode of action.
-
•
Lipid peroxidation, decreased metabolic activities, photosynthetic impairment was noted.
1. Introduction
Microplastics (MPs) are recognized as contributor to the biodiversity loss and can significantly affect the aquatic biota [1]. MPs can come to the environment from both primary and secondary sources. Polyethylene (PE) and polypropylene are the most often found polymers in water samples, followed by polystyrene (PS) and polycarbonate (PC), with microplastic concentrations ranging ten orders of magnitude (1ˑ10−5 - 1ˑ105 particles/liter) [2]. PS is derived from styrene monomer and is amorphous in nature. It has low specific weight, great transparency and minimal shrinkage. In addition to that it is also easy to manufacture industrially and can be used in various household materials and cosmetics [3]. It is available in several forms, including expanded polystyrene (EPS), general-purpose polystyrene (GPPS), high-impact polystyrene (HIPS) and extruded polystyrene (XPS) [4]. Personal care products, industrial abrasives, plastic pellets/flakes used as raw material in plastic fabrication are the main contributors to primary plastic pollution [5]. PS can also be generated from secondary sources due to degradation/fragmentation of larger plastics. These pieces come from fishing nets, films, fibers, industrial inputs, consumer goods, and household appliances [6]. The usage patterns indicate that the release of MPs in the aquatic habitats will rise over time and they will stay as persistent pollutants [7]. Once MPs enter the aquatic environment, they are subjected to various physical and chemical interactions including biofouling, weathering, and the integration of secondary pollutants. Depending on the characteristics, the plastic fragments are often found in various compartments of the aquatic ecosystem [8].
Phytoplankton is a major primary producer in freshwater food chain [9]. In freshwater system species richness, diversity, and abundance of phytoplankton play a critical role in maintaining the ecological balance. These indicators also provide necessary information regarding pollution level of particular aquatic ecosystem [9]. Previous studies reported that MPs would affect freshwater microalgae in concentration and size-dependent manner [10]. Sjollema et al., (2016) observed negative effects of uncharged and negatively charged microplastics of sizes 0.05, 0.5 and 6 µm on three different types of microalgae, D. tertiolecta, C. vulgaris, T. pseudonana [11]. Yokota et al. conducted a literature review that revealed primary producer–microplastic interactions can alter algal growth, photosynthetic efficiency, morphology, possibly via adhesion or transfer of adsorbed pollutants from microplastics. Wang et al., (2020) observed that both pristine and aged polyvinyl chloride microplastics (PVC MPs) would affect the growth rate and chlorophyll content of Chlamydomonas reinhardtii [12]. Xiao et al., (2020) investigated negative consequences of PS-MPs in freshwater microalgae, Euglena gracilis [13]. They noted that PS-MPs (0.1 and 5 µm) strongly suppressed algal growth in a concentration-dependent manner. Zhang et al., (2016) also revealed that PVC MPs inhibited the growth of Skeletonema costatum [14]. In another work [15] the impact of PS of varied surface charges in Scenedesmus obliquus. was assessed. The researchers reported reduced cell viability, photosynthetic efficiency and increased oxidative stress in the cells interacted with polystyrene nano plastics of 200 nm size with different surface charges. But they did not examine the effects of particles with different sizes or concentrations. Liu et al., (2019) reported, PS nano plastics (size: 100 nm) of different charges, (plain, aminated and carboxylated) caused growth inhibition and increase in the oxidative stress in S. obliquus [16]. But they did not explore the effects of varied sizes of PS nanoplastics on microalgae. Similarly, Huang et al., 2019 observed decreased cell viability and photosynthetic efficiency of S. obliquus upon interaction with three sizes of PS beads (0.1, 0.5, 1, and 2 µm) combined with different charges of PS (negative, positive and fluorescence tagged). But the size of PS-bead size was limited only to 2 µm. They did not study the effects of larger PS particles.
This necessitates a detailed study on the effects of both surface charge (plain, Aminated and Carboxylated) and size (lowest: 1 µm, highest: 12 µm) of MPs in freshwater microalgae. So, the major objective of the work was to explain the combined effects of size and surface charge of MPs in a freshwater alga, Scenedesmus obliquus. The major findings from this work will provide further cues in understanding the consequences of MP contamination in aquatic environment. A nuanced experimental approach has been taken to answer the following questions: (a) how polystyrene MPs of 1 and 12 µm sizes would affect growth, biochemical and photosynthetic parameters and (b) to what extent the toxic effects would depend on the surface charge of the particles (Plain-PS, COOH-PS, NH2-PS). The study involved assessment of growth parameters, photosynthetic and metabolic indicators, and oxidative stress parameters along with the activities of the crucial antioxidant enzymes.
2. Materials and methods
2.1. Chemicals
Polystyrene MPs of 1 and 12 µm size with different functionalization [Carboxyl-PS (COOH-PS); Aminated-PS (NH2-PS); Nonfunctionalized-PS (Plain-PS)] were purchased from Corpuscular, Inc., USA. Dihydroethidium (DHE) and Aminophenyl fluorescein (APF) were acquired from Invitrogen™, Molecular Probes®, CA, USA. Thiobarbituric acid (TBA), trichloroacetic acid (TCA), hydroxylamine hydrochloride, and dimethyl sulfoxide were purchased from Hi-Media Pvt. Ltd (Mumbai, India). Nitroblue tetrazolium chloride (NBT) and hydrogen peroxide solution (H2O2- 30% w/v) were procured from SDFCL, Mumbai, India. 2′,7′-Dichlorofluorescin diacetate (DCFDA), were purchased from Sigma–Aldrich Missouri (USA).
2.2. Preliminary characterization of PS in the exposure medium
Field Emission-Scanning electron microscope (FE-SEM) was used to determine and confirm the size and shape of PS. Aliquots of PS solution (1 mg/L) were kept on a piece of a glass slide, air-dried, sputter-coated with gold, and visualized through an electron microscope (operating voltage: 10.00 kV; working distance range: 9.4–10.7 mm) (Thermo Fisher FEI Quanta 250 FEG). To elucidate the charge on the MPs (1 and 12 µm), Zeta potential was evaluated at 0th h for all three differently functionalized MPs dispersed in lake water (90 Plus Particle Size Analyzer; software: Particle Size Analyzer, Brookhaven Instruments Corp., USA).
2.3. Culturing of Scenedesmus obliquus
The test organism used in this study was unicellular green freshwater algae, Scenedesmus obliquus, isolated from VIT lake, Vellore (12°58'10"N, 79°9'37"E). Sub-culturing and the maintenance of the Scenedesmus obliquus have been provided in the supplementary information (Method S1).
2.4. Assessing the cell toxicity in algae
2.4.1. Assessing cell viability
To determine the effects of two different sized and differently functionalized MPs, cell viability was evaluated by counting the cells. The detailed procedure has been provided in the supplementary information (Method S2).
2.4.2. Assessment of oxidative stress generated in algae
To estimate the oxidative stress generated in the control and treated algal cells, total ROS, superoxide radical, and hydroxyl radical generation were assessed. Followed by, antioxidant assays, Superoxide Dismutase (SOD) and Catalase were performed to determine the enzymatic activity in the algal cells. The total ROS production was measured using a cell membrane-permeable fluorescent dye, H2DCFDA [17] (Method S3). Dihydroethidium (DHE) ( is a membrane-permeable dye that has been used to monitor superoxide radical production in algal cells [18] (Method S4). Aminophenyl fluorescein (APF) is a dye that reacts with hydroxyl radicals to produce intense green fluorescence [19] (Method S5). The SOD assay was performed by the method described previously [20]. A series of chemical mixture (Na2CO3 buffer (50 mM, pH 10), 96 mM Nitro tetrazolium blue chloride (NBT), 0.6% Triton X-100, and 20 mM hydroxylamine hydrochloride) were used to perform SOD activity. The detailed protocol has been provided in the supplementary information (Method S6). Catalase assay was performed following the protocol mentioned in the previous report [21] (Method S7). To perform CAT activity 10.8 mM H2O2 solution (2 mL) and 50 mM - pH 7 potassium phosphate buffer (100 µL) was being treated with the algal supernatant. The detailed protocol has been provided in the supplementary information.
2.4.3. Estimation of photosynthetic parameters
The algal samples treated with PS were incubated in dark for 15 min. The dark-adapted samples (100 µL) were loaded into the Photosynthesis Yield Analyzer (Mini PAM, Heinz Walz, Germany). High-intensity actinic light was passed through the sample to record the ratio of variable fluorescence (Fv) to maximum fluorescence (Fm) of the sample [11]. This ratio is the effective photochemical quantum yield of PS II.
2.4.4. Metabolic activity
To assess the metabolic activity of the treated and control algal cells esterase and mitochondrial membrane potential assays were performed. According to the procedure reported elsewhere [22], fluorescein diacetate (FDA) was employed to quantify esterase activity and Rhodamine 123 (Rh123) was used to measure mitochondrial membrane potential (ΔΨm) [23]. The detailed protocol has been provided in the supplementary information (Method S8 and S9).
2.4.5. Determination of membrane integrity
To determine membrane integrity, lipid peroxidation (LPO) was performed and SYTOX green was used a fluorescence label. The LPO assay was used to determine the quantity of malondialdehyde (MDA), an end product of lipid peroxidation [24] (Method S10). The membrane integrity of PS-treated Scenedesmus sp. was determined using SYTOX green, a fluorescent nucleic acid binding dye that does not penetrate healthy cells [25]. The detailed protocol has been provided in the supplementary information (Method S11).
2.5. Statistical analysis
All the measurements were carried out in triplicate (n = 3) to demonstrate the statistical difference. Entire data is presented as mean ± standard deviation. To check the significant difference, Two-way ANOVA was performed with Bonferroni post-test using Graph Pad Prism 8.
3. Results
3.1. Characterization of PS
FE-SEM micrographs of 1 and 12 µm PS of different charges (plain, aminated and carboxylated) showed that all the MPs are spherical in shape (Supplementary Fig. S1). 1 µm PS showed the average size of 1.825 ± 0.011, 1.847 ± 0.007, and 1.851 ± 0.004 µm for Plain-PS, COOH-PS, and NH2-PS, respectively. Whereas 12 µm particles showed an average size of 10.603 ± 0.020, 13.046 ± 0.414, and 10.576 ± 0.194 µm for Plain-PS, COOH-PS, and NH2-PS, respectively. The surface charge details of the MPs dispersed in lake water are tabulated in Table 1.
Table 1.
Zeta Potential of differently functionalized and sized MPs dispersed in lake water medium.
| Dispersion Medium | Microplastics size | Charge on the MPs | Zeta Potential (mV) |
|---|---|---|---|
| Lake water | 1 µm | NH2-PS | -12.42 ± 0.65 |
| COOH-PS | -17.49 ± 2.04 | ||
| Plain-PS | -3.81 ± 1.1 | ||
| 12 µm | NH2-PS | -5.98 ± 0.27 | |
| COOH-PS | -6.92 ± 0.27 | ||
| Plain-PS | -0.78 ± 0.04 |
3.2. Toxicological responses in algae
3.2.1. Cell viability
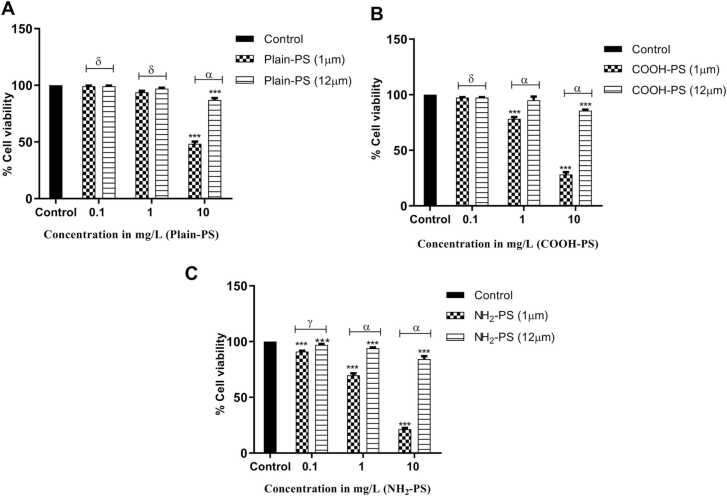
The toxicological effects of 1 and 12 µm PS in terms of cell viability are shown in Fig. 1. Upon exposure to increasing concentrations of PS (1 and 12 µm) dose-dependent effects at 1 and 10 mg/L was noted (p < 0.001) for all the three charges. The highest decrease in the cell viability was observed in NH2-PS treated algal cells followed by COOH-PS and Plain-PS.
Fig. 1.
Change in the cell viability of S. obliquus when compared to control (A) for Plain-PS interacted samples (n = 3), (B) for COOH-PS interacted samples (n = 3), (C) for NH2-PS interacted samples. Note: ‘α, γ, δ’ indicates significant difference represented significance between 1 and 12 µm MPs interacted samples (α = p < 0.001, γ = p < 0.05, δ = p > 0.05); ‘***’ indicates significant difference between test and control samples.
In case of 12 µm size, a significant difference compared to the control samples was found for 1 and 10 mg/L NH2-PS, 10 mg/L COOH-PS, and 10 mg/L Plain-PS treated samples (p < 0.001). When compared to 1 µm PS treated samples, cell viability in the algal cells treated with 12 µm PS significantly increased (p < 0.001) at 1 and 10 mg/L concentrations.
3.2.2. Oxidative stress: Total ROS, superoxide radical, hydroxyl radical
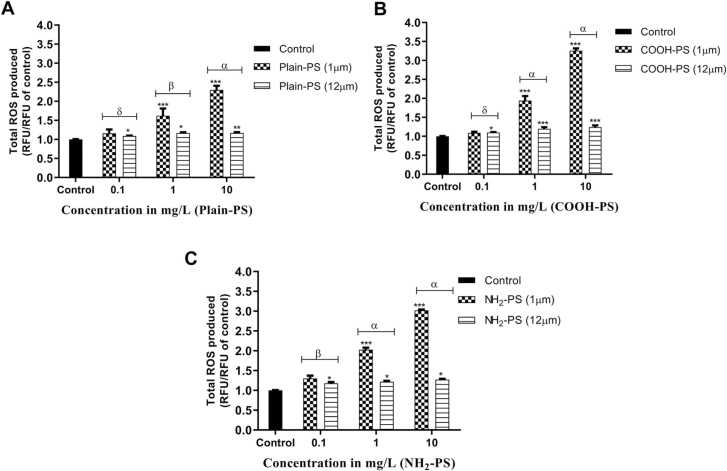
Fig. 2 illustrates the overall ROS levels in PS-treated algal samples. Based on the results, the highest ROS production was observed in 1 µm COOH-PS treated samples followed by NH2-PS and Plain-PS. It is observed that all the treatment groups experienced enhanced ROS levels (p < 0.001) in comparison with the control groups except for 0.1 mg/L PS treated cells. In 1 µm PS treated samples, a dose-dependent increase in ROS was found at all the charges of PS, and the difference between the surface charges was significant (p < 0.001).
Fig. 2.
Change in total ROS generation in S. obliquus relative to control (A) In case of Plain-PS interacted algae samples (n = 3), (B) In case of COOH-PS interacted algae samples (n = 3), (C) In case of NH2-PS interacted samples. Note: ‘α, β, δ’ indicates significant difference represented significance between 1 and 12 µm MPs interacted samples (α = p < 0.001, β = p < 0.01, δ = p > 0.05); ‘* ** ’, ‘* *’ and ‘* ’ indicates significant difference between test and control samples (‘***’ = p < 0.001, ‘**’ = p < 0.01, ‘*’ = p < 0.05).
When compared to 1 µm PS, 12 µm PS treated algal cells generated less ROS (Fig. 2). In this case, the highest ROS production was observed in COOH-PS treated samples followed by NH2-PS and Plain-PS. Furthermore, high significant differences in ROS generation between 1 and 12 µm PS treated samples was observed for 1 and 10 mg/L concentrations (p < 0.001).
Superoxide radical generation in response to 1 and 12 µm PS is depicted in Fig. 3. The highest generation was induced by NH2-PS followed by COOH-PS and Plain-PS. For 1 µm PS treated samples, concentration-dependent enhancement in generation was significant for all the charges of PS when compared to control (p < 0.001). Whereas for 12 µm PS treated samples, a significant difference (p < 0.001) compared to control was found only at the highest concentration of PS for all the functionalization. Comparing the two sizes of PS, a significant decrease in generation was observed for 12 µm PS in case of all the charges and their respective concentrations (p < 0.001).
Fig. 3.
Change in superoxide radical production in S. obliquus relative to control (A) in case of Plain-PS interacted algae samples (n = 3), (B) in case of COOH-PS interacted algae samples (n = 3), (C) In case of NH2-PS interacted algae samples. Note: ‘α’ indicates significant difference represented between 1 and 12 µm MPs interacted samples (α = p < 0.001); ‘***’ indicates significant difference between test and control samples.
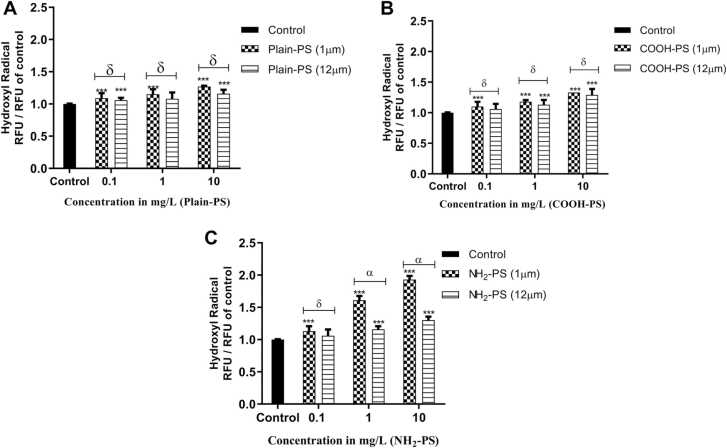
Fig. 4 shows the formation of hydroxyl radical in algal cells after treating with 1 and 12 µm PS. The highest hydroxyl radical generation was induced by NH2-PS followed by COOH-PS and Plain-PS. When compared to control, 1 µm PS treated samples showed a dose-dependent increase in hydroxyl radical generation, which was significant for all the charges of PS (p < 0.001). However, for 12 µm PS a significant drop in hydroxyl radical was noticed (p < 0.001) for all the surface charges across the test concentrations.
Fig. 4.
Change in hydroxyl radical production in S. obliquus relative to control (A) in case of Plain-PS interacted algae samples (n = 3), (B) in case of COOH-PS interacted algae samples (n = 3), (C) in case of NH2-PS interacted algae samples. Note: ‘α, δ’ indicates significant difference represented between 1 and 12 µm MPs interacted samples (α = p < 0.001, δ = p > 0.05); ‘***’ indicates significant difference between test and control samples.
3.2.3. Antioxidant enzyme activity
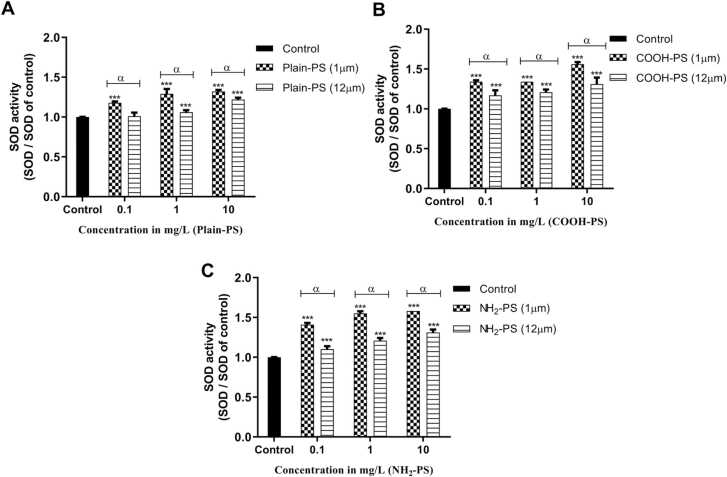
The activation of the SOD enzyme by 1 and 12 µm PS in algal cells is depicted in Fig. 5. The increase in enzyme activity was dose dependent. All the test groups demonstrated a significant increase in SOD activity when compared to the control (p < 0.001) except for 0.1 mg/L 1 µm Plain-PS treated algal cells (p > 0.05). When the charges and their corresponding concentrations are compared between 1 and 12 µm PS treated samples, a significant reduction in SOD activity is noticed in case of 12 µm PS (p < 0.001). For both the sizes of MPs, a significant difference in SOD activity was found between Plain-PS and NH2-PS across the concentration range (p < 0.001).
Fig. 5.
Change in SOD activity in S. obliquus relative to control (A) in case of Plain-PS interacted algae samples (n = 3), (B) in case of COOH-PS interacted algae samples (n = 3), (C) in case of NH2-PS interacted algae samples. Note: ‘α’ indicates significant difference represented between 1 and 12 µm MPs interacted samples (α = p < 0.001); ‘***’ indicates significant difference between test and control samples.
In case of CAT activity, both 1 and 12 µm PS treated samples demonstrated a significant increase concerning the control sets (p < 0.001) (Fig. S2). The highest CAT activity was observed in NH2-PS followed by COOH-PS and Plain-PS treated algal samples. When compared to 1 µm PS treated algal cells, the large decline in CAT activity in 12 µm PS treated algal cells was observed (p < 0.001). This difference was found for all three charges and their respective concentrations.
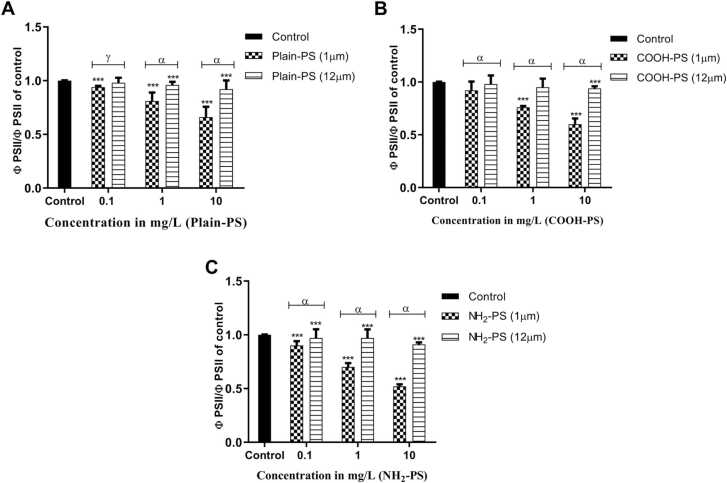
3.2.4. Effects on photosynthetic apparatus of algal cells: Maximum quantum yield of PS II and ETR
A clear dose-dependent response was noted in the yield of PS II after treating with the PS MPs (Fig. 6). A significant difference between the effects by Plain-PS and NH2-PS (p < 0.001) for all the concentrations was evident for 1 µm PS. In case of 12 µm PS treated samples, a significant decrease was noticed only in case of 1 and 10 mg/L concentrations. Likewise, when compared with 1 µm PS, 12 µm PS significantly enhanced the PS II yield at 1 and 10 mg/L concentrations for all the charges (p < 0.001). This enhancement in the yield was observed for NH2-PS followed by COOH-PS and Plain-PS (p < 0.001).
Fig. 6.
Change in Maximum quantum yield of PS II in S. obliquus relative to control (A) for Plain-PS interacted samples (n = 3), (B) for COOH-PS interacted samples (n = 3), (C) for NH2-PS interacted samples. Note: ‘α, γ’ indicates significant difference represented significance between 1 and 12 µm MPs interacted samples (α = p < 0.001, γ = p < 0.05); ‘***’ indicates significant difference between test and control samples.
A decrease in the electron transfer rate in the algal cells treated with 1 µm PS was significant at all the concentrations when compared to control (p < 0.001) (Fig. S3). However, 12 µm PS exhibited significant reduction only at 1 and 10 mg/L (p < 0.001). For both the sizes of MPs, a significant difference in ETR was found between Plain-PS and NH2-PS at all the concentrations (p < 0.001).
3.2.5. Effect on metabolic activity: Esterase activity and ΔΨm
The reduction in metabolic activity can be confirmed by a decline in FDA activity. The intensity of green fluorescence significantly decreased in the algal cells exposed to 1 µm PS, when compared to control (p < 0.001) (Fig. 7). However, such significant reduction in FDA fluorescence intensity was observed for 1 and 10 mg/L concentrations of 12 µm PS treated algal samples when compared to control. The increase in esterase activity in the algal cells treated with 12 µm PS was significant, when compared to 1 µm PS (p < 0.001).
Fig. 7.
Change in esterase activity in S. obliquus relative to control (A) for Plain-PS interacted samples (n = 3), (B) for COOH-PS interacted samples (n = 3), (C) for NH2-PS interacted samples. Note: ‘α’ indicates significant difference represented between 1 and 12 µm MPs interacted samples (α = p < 0.001); ‘***’ indicates significant difference between test and control samples.
The exposure to 1 µm PS provoked a significant reduction (p < 0.001) in Rh123 fluorescence at all the concentrations except 0.1 mg/L Plain-PS (Fig. S4). However, 12 µm PS treated algal cells revealed no significant reduction in Rh123 fluorescence when compared to the control cells (p > 0.05) except at the highest concentration i.e., 10 mg/L (p > 0.001). Moreover, hyperpolarization, i.e., an increase in ΔΨm was observed in 12 µm PS when compared to 1 µm PS at concentrations, 1 and 10 mg/L. This difference was also found to be significant (p > 0.001).
3.2.6. Effects on the membrane integrity
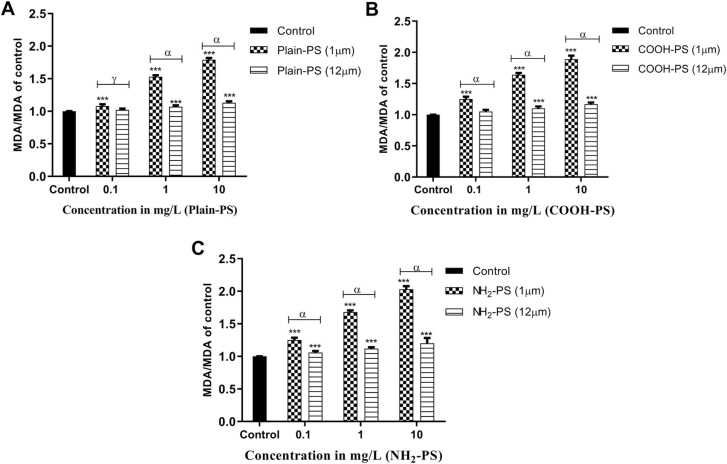
Fig. 8 displays the MDA content in algal cells interacted with 1 and 12 µm PS. Interaction with 1 µm PS produced a significantly higher amount of MDA for all the charges when compared to control (p < 0.001). The highest MDA content was observed in NH2-PS treated samples and the least production was noted in Plain-PS treated algal samples. When compared between two varied sizes, a significant reduction in MDA content can be noted in 12 µm PS for all the charges and their respective concentrations (p < 0.001). This again shows reduced toxic effects of 12 µm PS compared to 1 µm PS.
Fig. 8.
Change in lipid peroxidation in S. obliquus relative to control (A) for Plain-PS interacted samples (n = 3), (B) for COOH-PS interacted samples (n = 3), (C) for NH2-PS interacted samples. Note: ‘α, δ’ indicates significant difference represented significance between 1 and 12 µm MPs interacted samples (α = p < 0.001, δ = p > 0.05); ‘***’ indicates significant difference between test and control samples.
SYTOX Green was used to label the algal cells to distinguish the cells with damaged or permeabilized plasma membrane. The fluorescence intensity of the dye was stronger when exposed to 1 µm PS of three distinct charges of PS at all concentrations except for 0.1 mg/L Plain-PS, when compared to control (p < 0.001) (Fig. S5). Whereas 12 µm PS treated algal samples demonstrated a significant increase in fluorescence intensity only at higher concentrations when compared to control (p < 0.001). A significant difference in fluorescence intensity was observed between the two sizes for all the charges of MPs (p < 0.001).
4. Discussion
MPs pose deleterious effects to algae and may result in bio-accumulation and magnification, by entering the food chain [26]. To comprehend potential toxic effects of nanoparticles in aqueous test medium examining the effects of their surface charge is pertinent [27]. The low zeta potential values of the MPs suggest that the weak electrostatic repulsion between neighboring particles may lead to poor stabilization. So, they might tend to agglomerate.
PS-MPs of size 1 µm showed a dose dependent decline in cell viability irrespective of the charges. This may be due to the interaction of algal cell wall with the charged MPs. Adsorption of the particles on algae cell wall would damage the cell membrane facilitating particle internalization [28]. The MPs with effectively large surface area are readily ingested by algal cells resulting in toxicity [24]. MPs of size 12 µm did not exert any notable toxic effects on algae. Since algal cell walls have smaller pore sizes and are selectively permeable the entry of the large sized particles into the cells is restricted [29].
MPs irrespective of their sizes and charge are known to elicit oxidative stress in algae [11]. It is noted, 1 µm MPs generated more ROS compared to 12 µm MPs. Similar trends have been noticed for superoxide and hydroxyl radical generation too. Larger sizes of MPs tend to trigger lesser amount of oxidative stress than the smaller sized ones because small sizes can penetrate the cell membrane easily [30]. These reactive species can trigger damage to the cell membrane by interfering with the polyunsaturated fatty acids present in the algal cell membrane. This reveals a positive association between ROS production and lipid peroxidation [31]. In the previous studies 1 and 2 µm PS were found to be internalized by Platymonas helgolandica var. tsingtaoensis after 72 h of incubation but MPs of 3.0–5.0 µm size were found adhering to the cell surface only. The positively functionalized MPs (PS-NH2) caused more ROS production than negatively functionalized MPs (PS-COOH). Similar observations were confirmed by the previous researchers too [30], [32], [33].
MP-generated ROS enhances the activity of the antioxidant enzymes, SOD, and CAT in the cells, which serve as a defense mechanism to combat the oxidative stress [12]. Previous reports also revealed activation of anti-oxidant enzymes in Microcystis aeruginosa [34] and Euglena gracilis [13] after treating with MPs. SOD activity in the algal cells was more than the control sets for both 1 and 12 µm MPs treatment groups. CAT activity increased with the concentration of MP in the plain MP treated algal cells, but the activity was suppressed with an increasing concentration of PS-NH2. PS-COOH treated cells. An improved CAT activity suggests better amelioration of oxidative stress in the cells. Stress beyond the threshold of an organism’s tolerance levels can result in the decrease or even inhibition of the enzyme activity, leading to the accumulation of ROS and further oxidative damage to cells [35].
Photosynthesis is an important biochemical process of algae, which maintains the O2/CO2 balance and any disturbances in this process may affect the ecosystem adversely. Fv/Fm, a vital indicator for photosystem II (PSII) activity, is measured to identify stress-induced damage to the photosynthetic machinery. It is noted that induced oxidative stress impaired the photosynthetic machinery to a varied extent in algae. Previous research by Wu and colleagues showed that the Fv/Fm ratio declined with increasing nanoplastics concentration [36]. In this study, 1 µm MP reduced the quantum yield of PSII in a dose-dependent manner whereas 12 µm MP showed lesser damage to quantum yield. The electron transfer rate also followed a similar trend. It has been hypothesized that the PSNPs stress slows down the PS II electron transport rate. This causes an increased buildup of electrons, which in turn amplifies the photoinhibition and subsequent rise in reactive oxygen species (ROS). The production of ROS in the cells prevents the synthesis of chlorophyll, which results in a significant decrease in the yield of photosynthetic reactions [36]. In a previous study 1 mm PS-MPs exposure led to inhibition of the Fv/Fm ratio in C. pyrenoidosa [37]. Liu et al., 2020 reported that S. obliquus exposed to several types of polystyrene particles, (0.1, 0.5, 1, and 2 µm) showed a differential response to the size and charges of the particles.
Membrane integrity is strongly associated with the cell viability, which is regulated by mitochondrial and chloroplast functions [38]. It was observed that 1 µm PS led to greater reduction in mitochondrial membrane potential (MMP) than 12 µm PS. Enhanced MMP values in the 12 µm PS treated algal cells indicated enhanced mitochondrial function. Esterase activity is related to the algal cell metabolism. Loss of membrane integrity and decreased cell metabolism may lead to increased esterase activity [33], [39].
5. Conclusion
Plastic waste generation and its improper disposal are increasing every day, making MP pollution a chronic worldwide concern, more so for aquatic ecosystems. In the present context, this study has enormous potential to depict the differences in reaction of aquatic algae to MPs of varying size and charges. However, this study comes with certain impediments like, the MP uptake or translocation was not determined and the molecular mechanism underlying the stress response was not elucidated. This creates room for future investigations to focus on deciphering the underlying molecular framework and signaling events associated with MP induced stress and the long-term effect of MP deposition in the aquatic environment.
CRediT authorship contribution statement
Lokeshwari Natarajan: Investigation, Methodology, Visualization, Formal analysis, Writing – original draft. Soupam Das: Investigation, Methodology, Formal analysis. Swarnali Dey: Formal analysis, Writing – review and editing. N. Chandrasekaran: Formal analysis, Resources. Rita Kundu: Resources, Supervision, Writing – review and editing. Subhabrata Paul: Supervision, Investigation, Writing – review & editing. Amitava Mukherjee: Conceptualization, Supervision, Project administration, Writing – review and editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The research was supported by Department of Biotechnology, West Bengal [project no. 76 (Sanc.) – BT/P/Budget/RD-14/2017, Date 27.03.2017]. The authors are grateful to Department of Botany, University of Calcutta and School of Biotechnology, Presidency University for providing institutional and infrastructural support. SD (CSIR SPMF) is grateful to UGC for providing fellowship to him. Authors also would like to acknowledge Vellore Institute Technology (VIT), Vellore, India for the Field Emission Scanning Electron Microscopy facility used in this study.
Handling Editor: Dr. Prof. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.10.013.
Contributor Information
Subhabrata Paul, Email: subhabrata.dbs@presiuniv.ac.in.
Amitava Mukherjee, Email: amitav@vit.ac.in, amit.mookerjea@gmail.com.
Appendix A. Supplementary material
Supplementary material.
.
Data Availability
Data will be made available on request.
References
- 1.Gall S.C., Thompson R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015;92:170–179. doi: 10.1016/j.marpolbul.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Nugnes R., Lavorgna M., Orlo E., Russo C., Isidori M. Toxic impact of polystyrene microplastic particles in freshwater organisms. Chemosphere. 2022;299 doi: 10.1016/j.chemosphere.2022.134373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.S.A. Begum, A.V. Rane, K. Kanny, Chapter 20 - Applications of compatibilized polymer blends in automobile industry, in: A. A.R., S.B.T.-C. of P.B. Thomas (Eds.), Elsevier, 2020: pp. 563–593. https://doi.org/https://doi.org/10.1016/B978–0-12–816006-0.00020–7.
- 4.F. Galgani, G. Hanke, T. Maes, Global Distribution, Composition and Abundance of Marine Litter BT - Marine Anthropogenic Litter, in: M. Bergmann, L. Gutow, M. Klages (Eds.), Springer International Publishing, Cham, 2015: pp. 29–56. https://doi.org/10.1007/978–3-319–16510-3_2.
- 5.Duis K., Coors A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016;28:1–25. doi: 10.1186/s12302-015-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eerkes-Medrano D., Thompson R.C., Aldridge D.C. Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015;75:63–82. doi: 10.1016/j.watres.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Bi M., He Q., Chen Y. What roles are terrestrial plants playing in global microplastic cycling? Environ. Sci. Technol. 2020;54:5325–5327. doi: 10.1021/acs.est.0c01009. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S., Chatterjee S. Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ. Sci. Pollut. Res. 2017;24:21530–21547. doi: 10.1007/s11356-017-9910-8. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Fortún A., Fajardo C., Martín C., D’ors A., Nande M., Mengs G., Costa G., Martín M., Sánchez-Fortún S. Effects of polyethylene-type microplastics on the growth and primary production of the freshwater phytoplankton species Scenedesmus armatus and Microcystis aeruginosa. Environ. Exp. Bot. 2021;188 doi: 10.1016/j.envexpbot.2021.104510. [DOI] [Google Scholar]
- 10.Rani-Borges B., Moschini-Carlos V., Pompêo M. Microplastics and freshwater microalgae: what do we know so far? Aquat. Ecol. 2021;55:363–377. doi: 10.1007/s10452-021-09834-9. [DOI] [Google Scholar]
- 11.S.B. Sjollema, P. Redondo-hasselerharm, H.A. Leslie, M.H.S. Kraak, A.D. Vethaak, Do plastic particles affect microalgal photosynthesis and growth ?, 170 (2016) 259–261. [DOI] [PubMed]
- 12.Wang Q., Wangjin X., Zhang Y., Wang N., Wang Y., Meng G., Chen Y. The toxicity of virgin and UV-aged PVC microplastics on the growth of freshwater algae Chlamydomonas reinhardtii. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141603. [DOI] [PubMed] [Google Scholar]
- 13.Xiao Y., Jiang X., Liao Y., Zhao W., Zhao P., Li M. Adverse physiological and molecular level effects of polystyrene microplastics on freshwater microalgae. Chemosphere. 2020;255 doi: 10.1016/j.chemosphere.2020.126914. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C., Chen X., Wang J., Tan L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae *. Environ. Pollut. 2016:1–7. doi: 10.1016/j.envpol.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Giri S., Mukherjee A. Ageing with algal EPS reduces the toxic effects of polystyrene nanoplastics in freshwater microalgae Scenedesmus obliquus. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2021.105978. [DOI] [Google Scholar]
- 16.Liu Y., Wang Z., Wang S., Fang H., Ye N., Wang D. Ecotoxicological effects on Scenedesmus obliquus and Danio rerio Co-exposed to polystyrene nano-plastic particles and natural acidic organic polymer. Environ. Toxicol. Pharmacol. 2019;67:21–28. doi: 10.1016/j.etap.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Wang H., Joseph J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader11Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee by the United States Department of Agriculture and does not imp. Free Radic. Biol. Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 18.Owusu-Ansah E., Yavari A., Banerjee U. A protocol for in vivo detection of reactive oxygen species. Protoc. Exch. 2008:1–13. doi: 10.1038/nprot.2008.23. [DOI] [Google Scholar]
- 19.K. Setsukinai, Y. Urano, H.J. Majima, J.B. Chem, TRANSDUCTION: That Can Reliably Detect Reactive Oxygen Species and Distinguish Specific Species Supplemental material: Detect Reactive Oxygen Species and Distinguish Specific Species * □, (2003). https://doi.org/10.1074/jbc.M209264200. [DOI] [PubMed]
- 20.KONO Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. 1978;186:189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 21.Yilancioglu K., Cokol M., Pastirmaci I., Erman B., Cetiner S. Oxidative stress is a mediator for increased lipid accumulation in a newly isolated dunaliella salina strain. 2014;9 doi: 10.1371/journal.pone.0091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regel R.H., Ferris J.M., Ganf G.G., Brookes J.D. Algal esterase activity as a biomeasure of environmental degradation in a freshwater creek. Aquat. Toxicol. 2002;59:209–223. doi: 10.1016/S0166-445X(01)00254-5. [DOI] [PubMed] [Google Scholar]
- 23.Machado M.D., Lopes A.R., Soares E.V. Responses of the alga Pseudokirchneriella subcapitata to long-term exposure to metal stress. J. Hazard. Mater. 2015;296:82–92. doi: 10.1016/j.jhazmat.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Piotrowska-Niczyporuk A., Bajguz A., Zambrzycka E., Godlewska-Żyłkiewicz B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. PPB. 2012;52:52–65. doi: 10.1016/j.plaphy.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Feng L.J., Li J.W., Xu E.G., Sun X.D., Zhu F.P., Ding Z., Tian H., Dong S.S., Xia P.F., Yuan X.Z. Short-term exposure to positively charged polystyrene nanoparticles causes oxidative stress and membrane destruction in cyanobacteria. Environ. Sci. Nano. 2019;6:3072–3079. doi: 10.1039/c9en00807a. [DOI] [Google Scholar]
- 26.Van Raamsdonk L.W.D., Van Der Zande M., Koelmans A.A., Hoogenboom P.L.A., Peters R.J.B., Groot M.J., Peijnenburg M.A.C., Weesepoel Y.J.A. Current insights into monitoring, bioaccumulation, and potential health effects of microplastics present in the food chain. Foods. 2020;9 doi: 10.3390/foods9010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Tan Z., Peng J., Qiu Q., Li M. The behaviors of microplastics in the marine environment. Mar. Environ. Res. 2016;113:7–17. doi: 10.1016/j.marenvres.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Huang B., Wei Z.-B., Yang L.-Y., Pan K., Miao A.-J. Combined Toxicity of Silver Nanoparticles with Hematite or Plastic Nanoparticles toward Two Freshwater Algae. Environ. Sci. Technol. 2019;53:3871–3879. doi: 10.1021/acs.est.8b07001. [DOI] [PubMed] [Google Scholar]
- 29.D. Chakraborty, K.R. Ethiraj, N. Chandrasekaran, A. Mukherjee, Mitigating the toxic effects of CdSe quantum dots towards freshwater alga Scenedesmus obliquus: Role of eco-corona., Environmental Pollution (Barking, Essex: 1987). 270 (2021) 116049. https://doi.org/10.1016/j.envpol.2020.116049. [DOI] [PubMed]
- 30.Liu Z., Huang Y., Jiao Y., Chen Q., Wu D., Yu P., Li Y., Cai M., Zhao Y. Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat. Toxicol. 2020 doi: 10.1016/j.aquatox.2020.105420. [DOI] [PubMed] [Google Scholar]
- 31.C. Borza, Oxidative Stress and Lipid Peroxidation – A Lipid Metabolism Dysfunction, in: D. Muntean (Ed.), IntechOpen, Rijeka, 2013: p. Ch. 2. https://doi.org/10.5772/51627.
- 32.Bhattacharya P., Lin S., Turner J.P., Ke P.C. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J. Phys. Chem. C. 2010;114:16556–16561. doi: 10.1021/jp1054759. [DOI] [Google Scholar]
- 33.Natarajan L., Jenifer M.A., Chandrasekaran N., Suraishkumar G.K., Mukherjee A. Polystyrene nanoplastics diminish the toxic effects of Nano-TiO2 in marine algae Chlorella sp. Environ. Res. 2021 doi: 10.1016/j.envres.2021.112400. [DOI] [PubMed] [Google Scholar]
- 34.Zheng X., Zhang W., Yuan Y., Li Y., Liu X., Wang X., Fan Z. Growth inhibition, toxin production and oxidative stress caused by three microplastics in Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2021;208 doi: 10.1016/j.ecoenv.2020.111575. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012;2012:1–26. doi: 10.1155/2012/217037. [DOI] [Google Scholar]
- 36.Wu Y., Guo P., Zhang X., Zhang Y., Xie S., Deng J. Effect of microplastics exposure on the photosynthesis system of freshwater algae. J. Hazard. Mater. 2019;374:219–227. doi: 10.1016/j.jhazmat.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 37.Mao Y., Ai H., Chen Y., Zhang Z., Zeng P., Kang L., Li W., Gu W., He Q., Li H. Elsevier Ltd; 2018. Phytoplankton Response to Polystyrene Microplastics: Perspective from an Entire Growth Period. [DOI] [PubMed] [Google Scholar]
- 38.Chae Y., Kim D., An Y.-J. Effect of fluoride on the cell viability, cell organelle potential, and photosynthetic capacity of freshwater and soil algae. Environ. Pollut. 2016;219:359–367. doi: 10.1016/j.envpol.2016.10.063. [DOI] [PubMed] [Google Scholar]
- 39.González-Fernández C., Toullec J., Lambert C., Le Goïc N., Seoane M., Moriceau B., Huvet A., Berchel M., Vincent D., Courcot L., Soudant P., Paul-Pont I. Do transparent exopolymeric particles (TEP)affect the toxicity of nanoplastics on Chaetoceros neogracile? Environ. Pollut. 2019;250:873–882. doi: 10.1016/j.envpol.2019.04.093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.