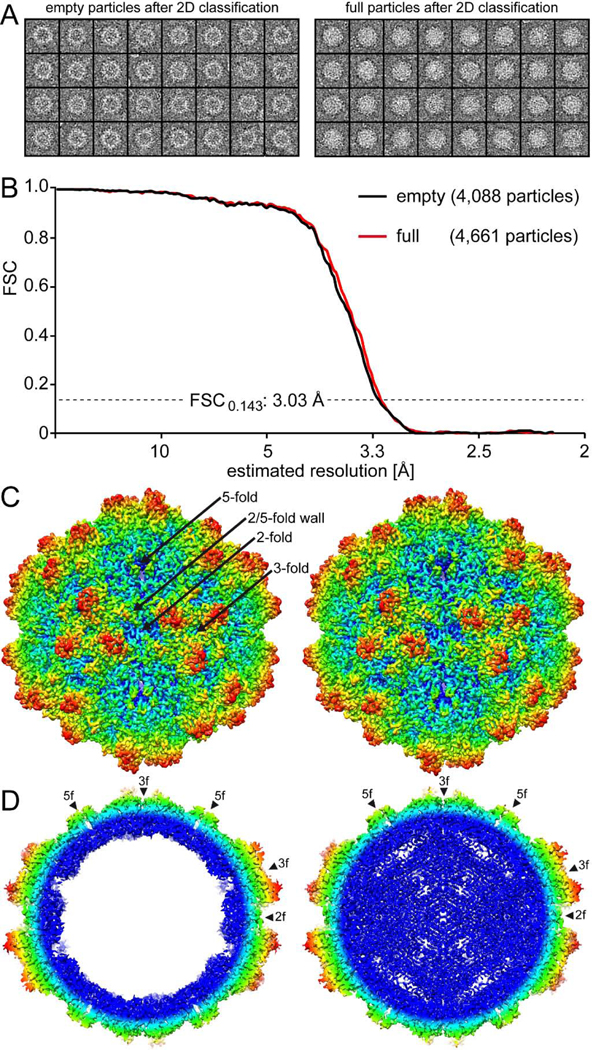
FIG. 2.
The cryo-EM reconstruction of the empty and full 10HB capsid structures. A) Separated empty and full particles following 2D-classification. B) Fourier Shell Correlation (FSC) plotted against resolution for the reconstructed 10HB structures. The resolution of the empty and full maps were estimated to be 3.03 Å, based on a FSC threshold of 0.143. C) The 10HB capsid surface density maps contoured at a sigma (σ) threshold level of 2.0. The maps are radially colored (blue to red) according to distance to the particle center, as indicated by the scale bar below. The icosahedral 2-, 3-, and 5-fold axes and the 2/5-fold wall are indicated on the empty 10HB capsid map. D) Cross-sectional views of the maps from the empty and full particles contoured at 1.0σ. The positions of the icosahedral 2-, 3-, and 5-fold symmetry axes are indicated by arrowheads. This figure was generated using UCSF-Chimera (Pettersen et al., 2004).