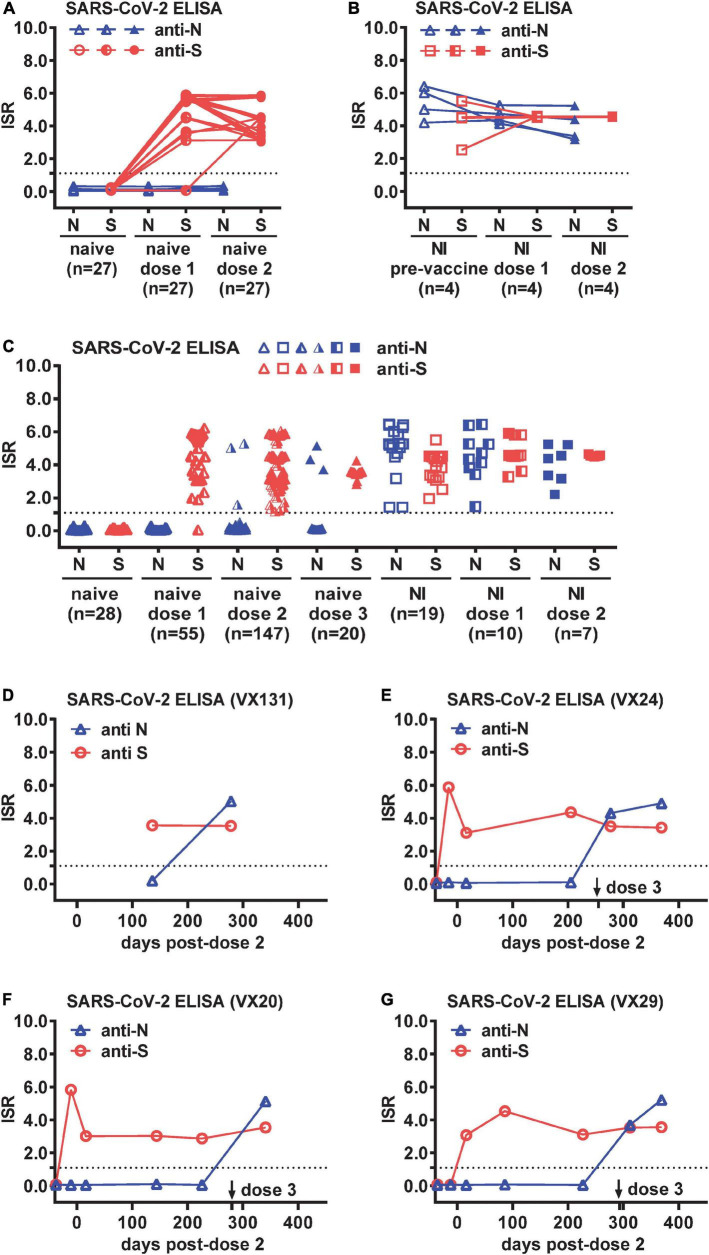
FIGURE 1.
Breakthrough infections (BTIs) identified by anti-N and anti-S ELISAs among COVID-19-naïve vaccinees. Results of anti-N and anti-S ELISAs of sequential plasma samples from (A) COVID-19-naïve (n = 27) and (B) SARS-CoV-2 natural infection (NI) (n = 4) panels before and after one and two doses of a mRNA vaccine (Moderna or Pfizer). (C) Results of anti-N and anti-S ELISAs of single or sequential plasma samples from COVID-19-naïve participants following one (n = 55), two (n = 147), or three (n = 20) doses of a mRNA vaccine, and controls from COVID-19-naïve (n = 28), NI (n = 19), and NI followed by one (n = 10) or two (n = 7) doses of a mRNA vaccine. (D–G) Results of anti-N and anti-S ELISAs of sequential plasma samples from four COVID-19-naïve participants (VX131, VX24, VX20, VX29) with a BTI identified by anti-N antibody seroconversion following two or three doses of Moderna vaccine. Dotted lines indicate the cut-off of immunological status ratio (ISR) values for ELISAs. Data are the mean of duplicates from one experiment.