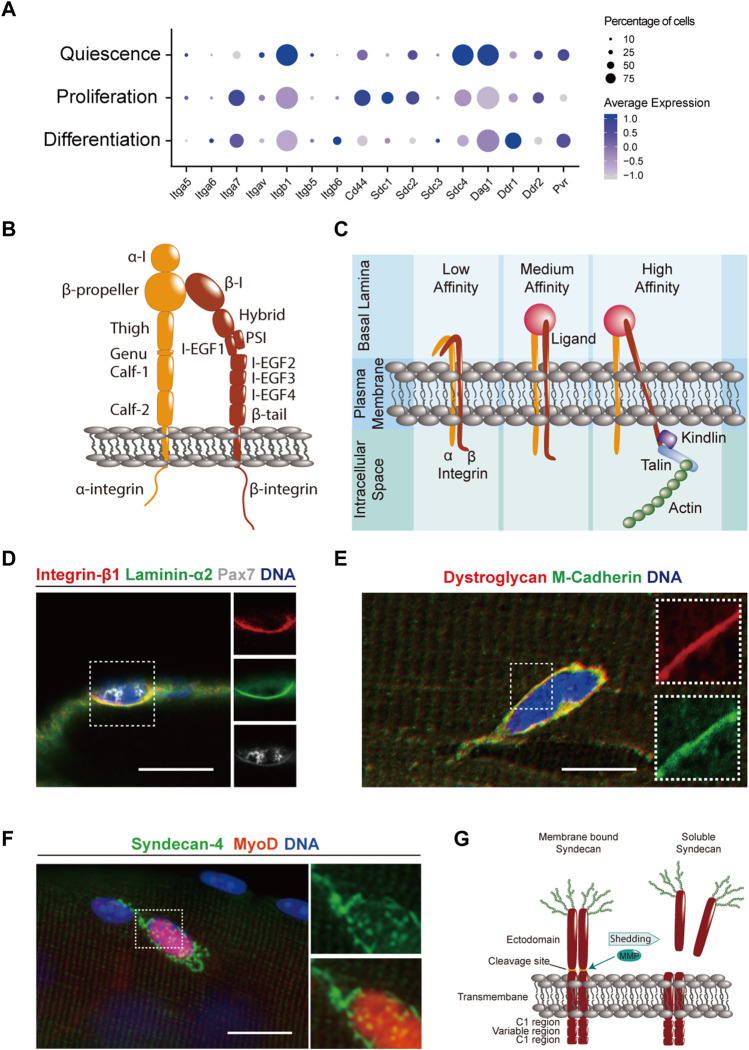
FIGURE 2.
Transmembrane ECM components and receptors in MuSCs. (A) Dot plot of single cell RNA-sequencing data (Oprescu et al., 2020) obtained from the Gene Expression Omnibus (GEO) database (Barrett et al., 2013) from uninjured and regenerating skeletal mouse muscle at 5 days post injury that was generated using Seurat 4.0.5. (Hao et al., 2021). Genes coding for ECM interacting transmembrane proteins were selected based on the GO terms “adhesion receptor function” in the adhesome data base and “ECM receptors” in the reactome data base (Zaidel-Bar et al., 2007; Winograd-Katz et al., 2014; Griss et al., 2020). The data for quiescence was obtained from uninjured muscles, while proliferation and differentiation were defined based on the presence or absence of expression of genes such as Pax7, MyoD, MyoG, and MKI67 at 5 days post injury. Only genes significantly expressed by at least 10% of MuSCs are shown. (B) Scheme showing the domain structure of the integrin-α and -β subunits. (C) Scheme illustrating how integrins transition from their low-to high-affinity state upon multivalent ligand binding and intracellular stabilization by focal adhesion molecules. (D) Immunostaining showing integrin β1 (red), laminin α2 (green), Pax7 (white), and DNA (blue) in a MuSC on a manually teased mouse muscle fiber bundle preparation. (E) Immunostaining showing dystroglycan (red), M-cadherin (green), and DNA (blue) in a MuSC on an enzymatically isolated single mouse muscle fiber. (F) Immunostaining showing syndecan-4 (green), MyoD (red) and DNA (blue) in MuSCs on an enzymatically isolated single mouse muscle fiber after 24 h of culture. (G) Scheme showing the domain structure of syndecan dimers in their membrane bound and soluble forms. Matrix metalloproteinases (MMPs) cleave membrane syndecans in a process called “shedding” and release the extracellular domain into the surrounding ECM. Scale bars = 20 μm (D,E), and 10 μm (F).