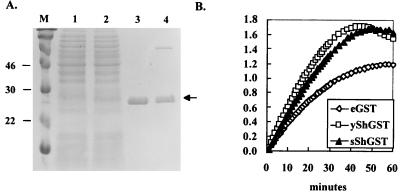
FIG. 5.
Purification and enzymatic activity of the Sh28GST expressed by JJ704 harboring pTECH10-Sh28. (A) Sh28GST was purified by single-step affinity chromatography on a GSH-agarose column. Purified proteins were subjected to SDS-PAGE and Coomassie blue staining. Lane 1, whole-cell lysate of SL3261; lane 2, whole-cell lysate of JJ704 harboring pTECH10-Sh28; lane 3, purified Sh28GST produced in JJ704; lane 4, purified Sh28GST produced in yeast (∼2 μg). Arrow, position of Sh28GST. The molecular mass markers are shown in lane M, and their sizes in kilodaltons are at the left. (B) GST activity catalyzed by Sh28GST purified from yeast (open squares), S. enterica serovar Typhimurium strain typhimurium JJ704 (solid triangles), and commercially purified GST of equine origin (Sigma; open diamonds) in a time-dependent fashion.