Figure 5.
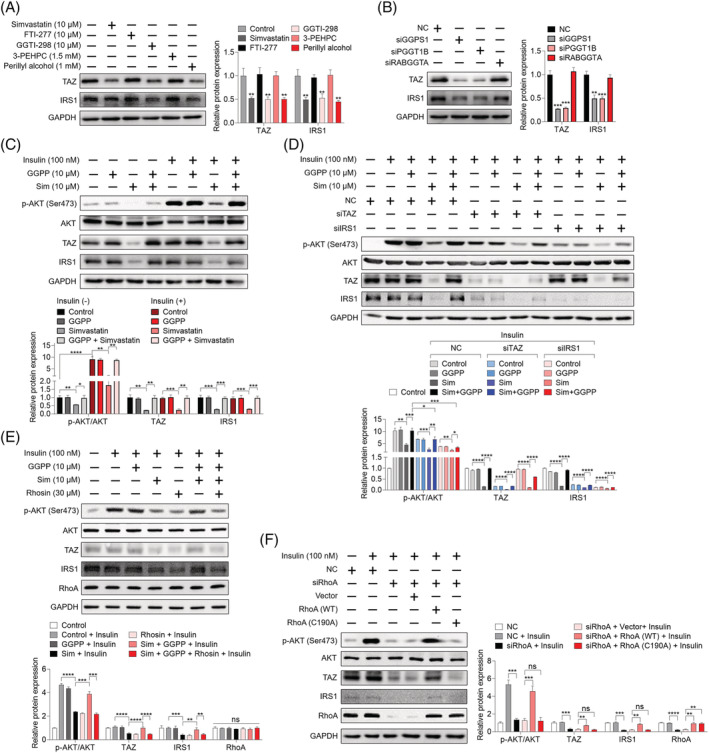
Geranylgeranyl pyrophosphate (GGPP) reverses simvastatin‐caused inhibition of insulin signalling via recovering RhoA geranylgeranylation‐mediated TAZ/IRS1 axis. (A) C2C12 myotubes were treated with 10 μM simvastatin, 10 μM FTI‐277, 10 μM GGTI‐298, 1.5 mM 3‐PEHPC, and 1 mM perillyl alcohol for 24 h, protein samples were harvested, and the expression of indicated proteins was analysed by western blot, with GAPDH as the loading control (n = 3). (B) C2C12 myotubes were transfected with siRNAs specifically targeting GGPS1, PGGT1B, and RABGGTA, respectively, for 48 h. Protein samples were harvested, and the expression of indicated proteins was analysed by western blot, with GAPDH as the loading control (n = 3). (C) C2C12 myotubes were treated with 10 μM GGPP, 10 μM simvastatin, or 10 μM GGPP combined with 10 μM simvastatin for 24 h. Then cells were treated or not treated with 100 nM insulin for 30 min. Protein samples were harvested, and the expression of indicated proteins was analysed by western blot, with GAPDH as the loading control (n = 3). (D) C2C12 myotubes were previously transfected with NC or siRNA specifically targeting TAZ and IRS1, respectively, for 48 h. Then cells were treated with 10 μM GGPP, 10 μM simvastatin, or 10 μM GGPP combined with 10 μM simvastatin for another 24 h. Before the end of the experiment, cells were incubated with 100 nM insulin for 30 min. Then protein samples were harvested, and the expression of indicated proteins was analysed by western blot, with GAPDH as the loading control (n = 3). (E) C2C12 myotubes were treated with 10 μM GGPP, 10 μM simvastatin and 30 μM Rhosin as indicated for 24 h. Then cells were incubated with 100 nM insulin for 30 min, and the expression of indicated proteins was analysed by western blot, with GAPDH as the loading control (n = 3). (F) C2C12 myotubes were previously transfected with siRNA targeting RhoA for 24 h, and then cells were transfected with vector, RhoA (WT), or RhoA (C190A) plasmids for 48 h. Before the end of the experiment, cells were incubated with 100 nM insulin for 30 min, then total protein samples were harvested, and the expression of indicated proteins was analysed by western blot, with GAPDH as the loading control (n = 3). Data represented the mean ± SEM. Statistical analysis was performed with one‐way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
