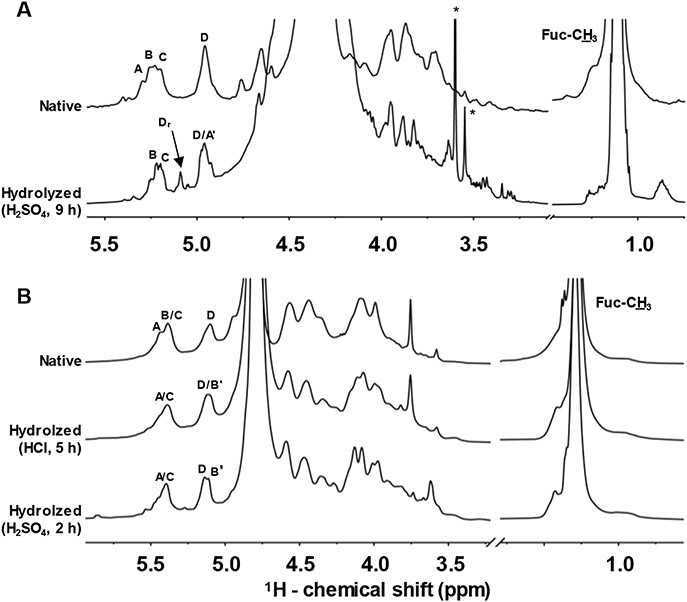
Fig. 3.
1D 1H NMR spectra of (A) native (top) and hydrolyzed IbSF (0.05 mol/dm3 H2SO4 at 60 °C for 9 h) (bottom), and (B) native (top), and hydrolyzed HfSF (0.01 mol/dm3 HCl at 60 °C for 5 h) (middle), and (0.05 mol/dm3 H2SO4 at 60 °C for 2 h) (bottom) recorded in 99% D2O at 50 °C on an AVANCE III 500 MHz Bruker NMR spectrometer equipped with a prodigy probe. 1H chemical shifts are referenced to trimethylsilylpropionic acid to 0 ppm. The 1H resonances marked as A-D in the native sulfated fucans are related to the fucose (Fuc) units labeled in Fig. 1. The 1H resonances assigned as A' and B' in the oligosaccharides are from desulfated Fuc units. The signal assigned as Dr is from the reducing-end 4-sulfated Fuc unit (D) in IbSF. Sharp signals marked with asterisks are from residual solvent during oligosaccharide production.