Fig. 1.
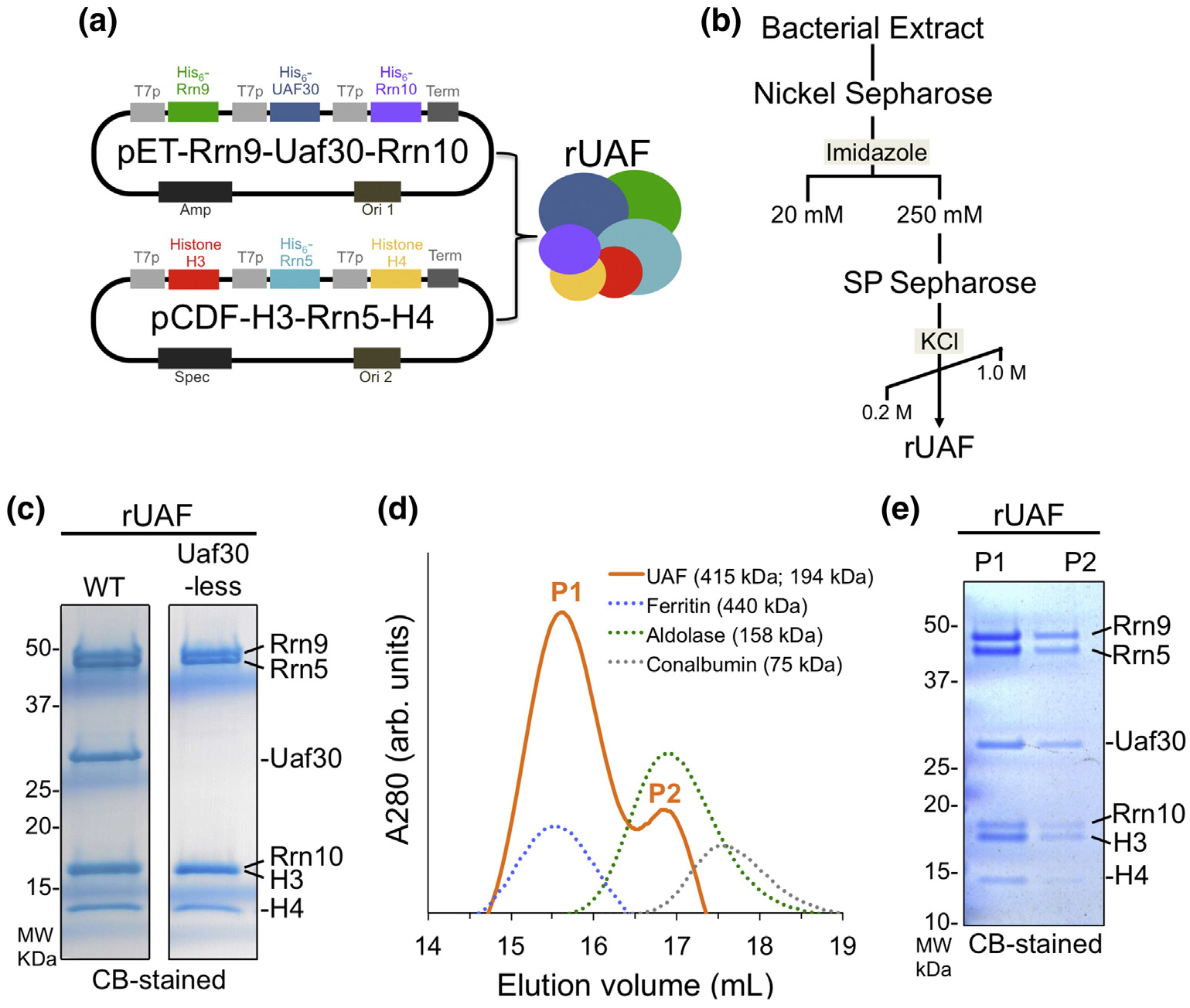
Expression and purification of rUAF. (a) Diagram of the two T7 bacteria expression vectors used to coexpress rUAF complex. T7P, T7 promoter; Term, T7 terminator; amp, ampicillin; spec, spectinomycin; ori, origin of replication. (b) rUAF purification scheme. (c) Coomassie blue (CB)-stained SDS-PAGE gel of purified rUAF (left) and rUAF lacking the Uaf30 subunit (right). Note that Rrn10 and histone H3 resolved as a single band on a 4%–20% Tris–glycine gel. MW, molecular weight. (d) SEC of purified rUAF. Elution profile of UAF (solid orange line) and standards (dashed line). Estimated molecular weight of UAF peaks and standards are indicated. P1/P2, peak 1/2; arb. units, arbitrary units. (e) CB-stained Bis–Tris SDS-PAGE gel of UAF peaks 1 and 2.
