Fig. 6.
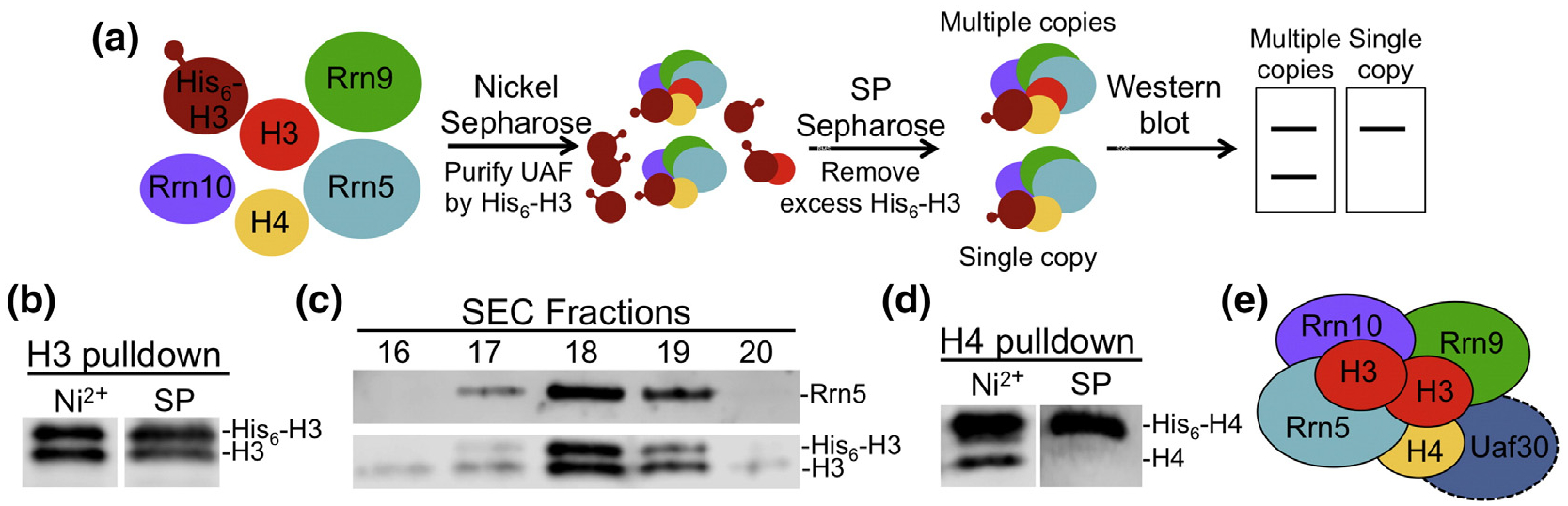
Stoichiometry of histone H3 and H4 subunits in UAF. (a). Schematic representation of the pulldown method used to determine histone H3 stoichiometry. UAF subunits including both a tagged and nontagged version of histone H3 were coexpressed and purified by Nickel sepharose followed by SP sepharose. Western blot analysis was then used to determine if both the tagged and nontagged forms of H3 were present in purified UAF. Western blot analysis of the Nickel sepharose and SP sepharose elutions of (b) H3 pulldowns and (d) H4 pulldowns probed using anti-histone H3 or H4 antibodies (c). UAF SEC fractions were analyzed by Western blot using antibodies against Rrn5 and histone H3 (e). UAF forms a stable complex with two copies of histone H3, and one copy of histone H4. These histone proteins combine with the putative histone fold domain of Rrn5 to form a hybrid tetramer-like core complex. The remaining UAF-specific subunits, Rrn10, Rrn9, and Uaf30 envelop the tetramer-like core complex.
