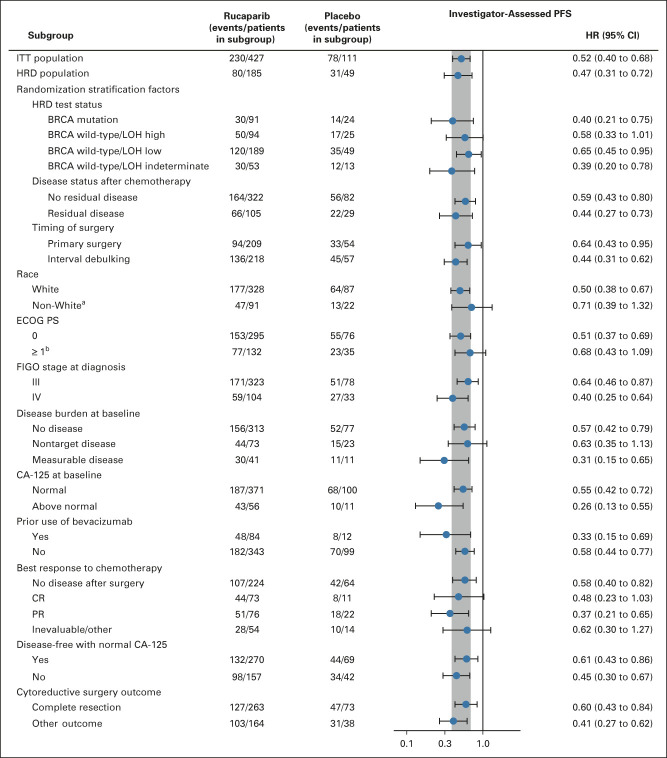
FIG 3.
Investigator-assessed PFS in subgroups in the ITT population. The vertical gray band corresponds to the 95% CI of the ITT population. aExcludes patients with unknown race. bOne rucaparib-treated patient had an ECOG PS of 1 at screening and 2 at cycle 1 day 1. BRCA, BRCA1 or BRCA2; CA-125, cancer antigen 125; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; HRD, homologous recombination deficiency; ITT, intent-to-treat; LOH, loss of heterozygosity; PFS, progression-free survival; PR, partial response.