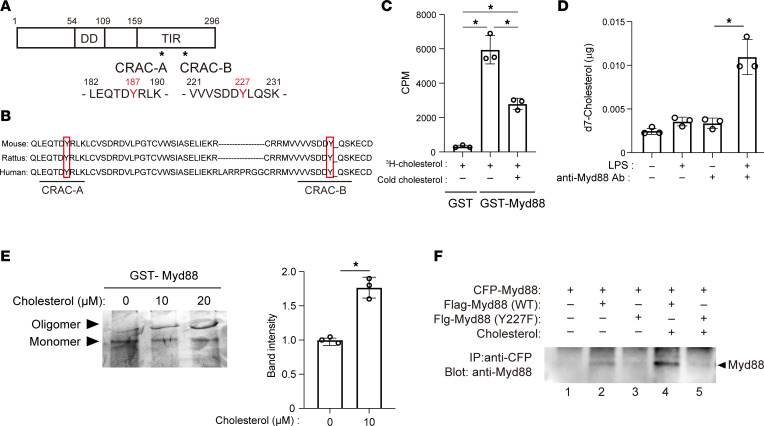
Figure 4. Cholesterol binds to the Myd88 CRAC domain.
(A) Schematic representation of mouse Myd88 protein. There are 2 CRAC sequences in its C-terminal Toll/interleukin 1 receptor (TIR) domain. DD, death domain. (B) Myd88 amino acid sequences around 2 CRAC sequences in human, rat, and mouse. (C) Affinity-purified, recombinant, GST-tagged Myd88 or GST was incubated with 3H‑cholesterol (10 μM). For the competition experiment, unlabeled cholesterol (10 μM) was added. GST-Myd88 and GST were recovered, and radioactivity was measured. *P < 0.05. Tukey-Kramer post hoc test. (D) RAW cells were cultured for 24 hours in medium containing deuterium-labeled cholesterol (d7-cholesterol) (10 μg/mL), with or without 4 hours of LPS stimulation. Whole-cell lysates were then extracted, and the amount of d7-cholesterol pulled down by Myd88 antibody was detected with GC/MS. *P < 0.05 vs. LPS-untreated cell lysates pulled down with anti-Myd88 antibody. (E) Purified GST-Myd88 was incubated with cholesterol, run on a Native polyacrylamide gel, and visualized by silver staining. Relative band intensities corresponding to oligomeric Myd88 are shown in the bar graph. n = 3 in each group. *P < 0.05. Student’s 2-tailed t test. (F) BMDMs were transfected with plasmids expressing Flag-tagged WT or Y227F mutant Myd88 along with CFP-tagged Myd88 and treated with or without cholesterol (10 μM) for 4 hours. Whole-cell lysates were collected and subjected to immunoprecipitation analysis. Data shown are representative of 3 independent experiments. Data shown as mean ± SD in all panels where P values are shown.