Figure 2.
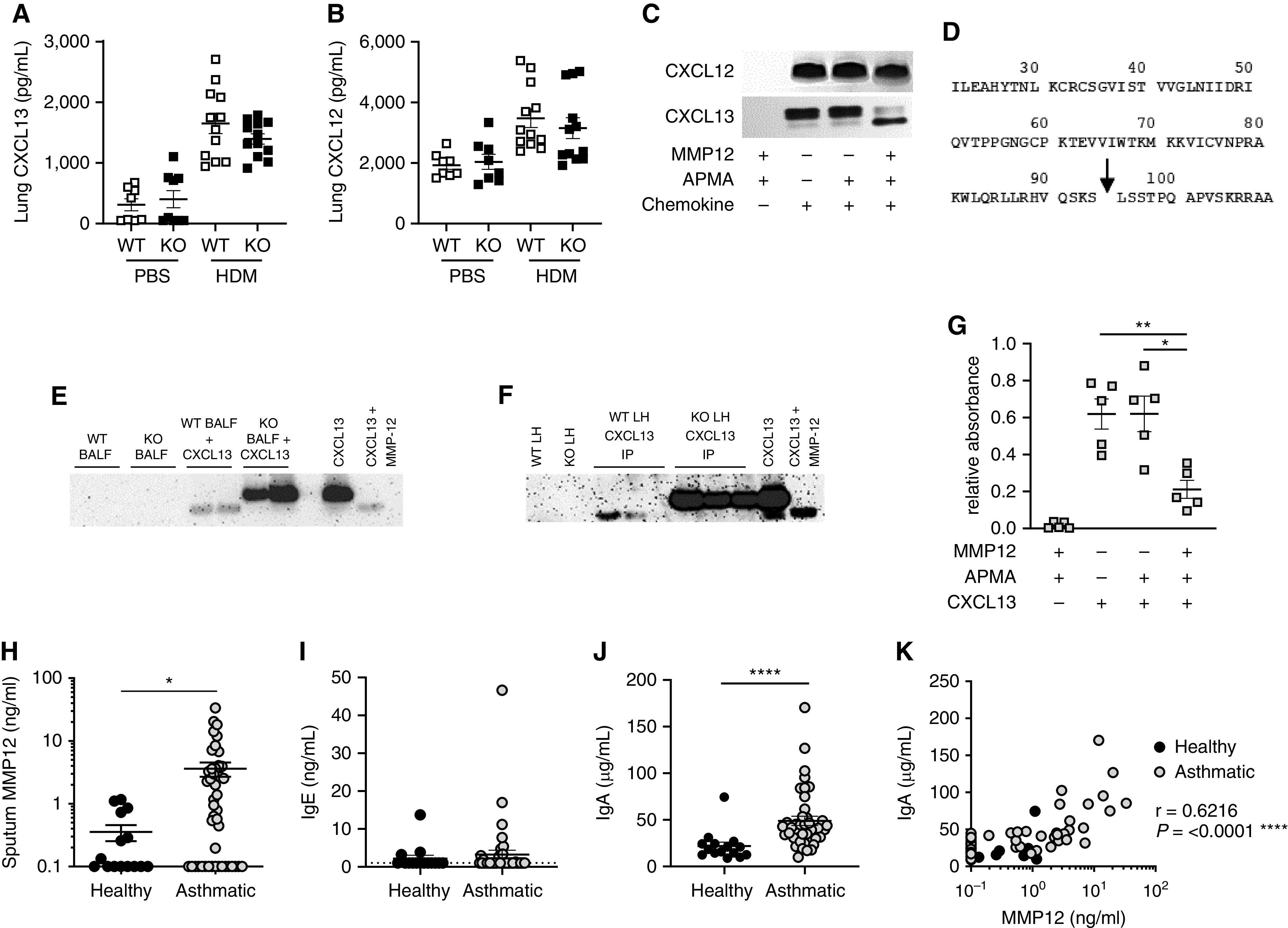
MMP-12 (matrix metalloproteinase-12) proteolytically processes CXCL13 to modulate glycosaminoglycan binding and correlates with airway antibody responses in patients with asthma. (A and B) The concentrations of CXCL13 (A) and CXCL12 (B) within lung homogenate (5) were measured by ELISA (R&D Systems). (C) Recombinant mouse CXCL13 and CXCL12 (both 100 μg/ml; Peprotech) were incubated with APMA (4-Aminophenylmercuric acetate)-activated (Sigma Aldrich) recombinant mouse MMP-12 (10 μg/ml; Abcam) for 8 hours at 37°C. The reaction products were separated by gel electrophoresis using NuPAGE 4–12% Bis-Tris Protein Gels (Thermo Fisher) and detected using Pierce Silver Stain kit. (D) Identification of murine MMP-12 cleavage products by an Applied Biosystems 4800 MALDI-TOF mass spectrometer in linear mode; MMP-12 cleavage site within murine CXCL13 denoted by arrow. (E) BAL fluid (BALF) from HDM-exposed wild-type (WT) or Mmp12−/− (KO) mice was incubated with CXCL13 (50 ng; Peprotech) for 16 hours at 37°C. The reaction products were separated by gel electrophoresis using NuPAGE 4–12% Bis-Tris Protein Gels (Thermo Fisher). Comparable amounts of WT and MMP-12 KO BALF alone or recombinant CXCL13 alone (with or without MMP-12 cleavage as described above) were run as controls. Proteins were transferred to PVDF (polyvinylidene fluoride) membrane and probed with biotinylated anti-CXCL13 antibody (0.2 μg/ml; R&D Systems), followed by Streptavidin-HRP conjugate (R&D Systems). (F) Immunoprecipitations (IPs) were performed on pooled lung homogenate (LH) from HDM-exposed WT or MMP-12 KO mice using biotinylated anti-CXCL13 antibody (10 μg; R&D Systems) and Streptavidin Dynabeads (Invitrogen). Samples were interrogated by Western blot as detailed above. WT and MMP-12 KO LH or recombinant CXCL13 (with or without MMP-12 cleavage as defined above) were run as controls. (G) CXCL13–MMP-12 reaction products (10 pmol CXCL13/well) were assessed for their capacity to bind biotinylated heparan sulfate (Sigma). (H–K) Analysis of sputum samples from a cohort (5) of 15 healthy volunteers and 42 patients with moderate to severe asthma (defined by British Thoracic Society guideline criteria [disease steps 3–5]; Research Ethics Committee Reference 10/H1010/7). (H) Sputum MMP-12 measured by ELISA (Abcam). (I and J) Sputum IgE (I) and IgA (J) measured by ELISA (Invitrogen). (K) Correlation between sputum MMP-12 and IgA. Solid symbols represent healthy control subjects; open symbols represent patients with asthma. Results depicted as mean ± SEM. (A, B, G–J) *P < 0.05, **P < 0.01, and ****P < 0.0001 using Mann-Whitney statistical test. Correlation analysis (K) was performed using Spearman rank test.
