Abstract
COVID-19 has altered many aspects of everyday life. For the scientific community, the pandemic has called upon investigators to continue work in novel ways, curtailing field and lab research. However, this unprecedented situation also offers an opportunity for researchers to optimize and further develop available field methods. Camera traps are one example of a tool used in science to answer questions about wildlife ecology, conservation, and management. Camera traps have long battery lives, lasting more than a year in certain cases, and photo storage capacity, with some models capable of wirelessly transmitting images from the field. This allows researchers to deploy cameras without having to check them for up to a year or more, making them an ideal field research tool during restrictions on in-person research activities such as COVID-19 lockdowns. As technological advances allow cameras to collect increasingly greater numbers of photos and videos, the analysis techniques for large amounts of data are evolving. Here, we describe the most common research questions suitable for camera trap studies and their importance for biodiversity conservation. As COVID-19 continues to affect how people interact with the natural environment, we discuss novel questions for which camera traps can provide insights on. We conclude by summarizing the results of a systematic review of camera trap studies, providing data on target taxa, geographic distribution, publication rate, and publication venues to help researchers planning to use camera traps in response to the current changes in human activity.
Keywords: COVID-19, Camera traps, Conservation biology, Data analysis, Biodiversity monitoring, Wildlife ecology, Remote sensing, Tropical biology
1. Introduction
The spread of COVID-19 across the globe has led to lockdowns of cities, towns, villages, and protected areas, resulting in drastic alterations to human activity. Most notably, these restrictions have led to extensive decreases in human mobility, a period some are calling the “anthropause” (Rutz et al., 2020). However, this change in human activity across the globe has not been consistent, and there exists wide variation in how certain locales have responded to the pandemic (Bennett et al., 2020; Rutz et al., 2020; Zellmer et al., 2020). Specifically, the extent to which local governments enforce restrictions varies by country or municipality, resulting in a gradient of human activity and mobility changes (Kleinschroth and Kowarik, 2020; Rutz et al., 2020). This combination of overall decreases in human mobility and natural variation in human traffic across different geographies, specifically in cities and natural areas, offers a unique opportunity for scientists to study and understand human-wildlife interactions on an unparalleled scale (Bates et al., 2020; Corlett et al., 2020; Saraswat and Saraswat, 2020).
However, scientific research is also susceptible to the effects of lockdowns. Researchers have been forced to sideline, cancel, or postpone their projects as a result of the COVID-19 restrictions (Pennisi, 2020). For many, long-term monitoring projects have been put on hold, field research grants have been postponed, and travel has been restricted. With the number of cases of COVID-19 continuing to grow worldwide and extending these restrictions indefinitely, the need for projects that allow monitoring without extensive time in the field, human involvement, and regular maintenance has never been more urgent. Camera traps provide a useful method for conservation ecologists to both continue important research and to investigate the novel wildlife conservation and ecology questions posed by this current pandemic.
Camera traps, or trail cameras, are motion- and heat-activated remote sensing devices that come in a wide variety of settings and are equipped with myriad triggering mechanisms, photo/video capabilities, sensor levels, flash types, housing, and other specifications (Rovero et al., 2013). The majority of modern cameras use a passive infrared sensor (PIR) that can detect the differences in heat and motion (Rovero et al., 2013). Most camera traps are relatively low-cost tools for research and management, with negligible impacts on target species or the environment (O'Connell et al., 2011; Burton et al., 2015; Steenweg et al., 2017; Caravaggi et al., 2017; Wearn and Glover-Kapfer, 2019).
Over the past couple of decades, the use of camera traps in conservation biology, ecology, and biodiversity assessments has grown significantly (McCallum, 2013; Burton et al., 2015; Steenweg et al., 2017; Kays et al., 2020). However, without appropriate research design, advanced planning, and power analyses, conservation biologists often collect high volumes of data that they are unable to use to either inform the management of vulnerable species and systems or to answer the conservation questions that initiated their research (Hebblewhite and Haydon, 2010). Since camera traps are relatively easy to setup and maintain in the field, researchers may inherit a false sense of security with their use as scientific research tools and neglect important aspects of the study design process. For efficient use of time and resources, researchers must distinguish the reasons for camera trapping research programs before deployment and choose appropriate study designs and analyses for conservation monitoring programs (Jones et al., 2013; Wearn and Glover-Kapfer, 2019; Kays et al., 2020).
In this review, we summarize the most common camera trap objectives, specifically: 1) documenting species presence/richness, 2) evaluating relative abundance, 3) estimating density, 4) estimating occupancy, and 5) quantifying activity patterns. We discuss the appropriate use of camera traps for each of these objectives and illustrate how such studies are designed and conducted, as well as how the resultant data are analyzed. We then provide, within each objective, key wildlife conservation and ecology questions associated with the current “anthropause” (Rutz et al., 2020) that camera traps are well-suited to tackling. We conclude by providing results from a systematic review of camera trap studies, providing information on publication rate, popular publication venues, target taxa, and geographic distribution of studies worldwide. We also provided a summary on current camera trap initiatives both actively gathering data and looking for additional collaborators. Our goal is to inform conservation biologists of the advantages and disadvantages of camera traps; assist in the appropriate choice of method and study design; discuss how camera traps can be critical for studying wildlife behavior, activity, and dispersal in the time of COVID-19; and review the contribution of camera trapping studies to conservation biology.
1.1. Biological objectives of camera trap studies
Camera traps have been used to study many species and objectives in animal ecology (O'Connell et al., 2011; Burton et al., 2015). Here we focus on the most common study objectives: presence, relative abundance, density, occupancy, and activity. Early in the use of camera traps for research purposes, few studies went beyond baseline assessments of population size and structure (Linkie et al., 2010). However, conservationists are increasingly using camera traps to test hypotheses and address a range of questions including human impacts on wildlife (Main and Richardson, 2002; Magle et al., 2012; Gallo et al., 2017; Parsons et al., 2018; Fidino et al.,2020; Parsons et al., 2019), biodiversity monitoring over space and time (Waldon et al., 2011; Gilbert et al., 2020), reproductive ecology (Farhadinia et al., 2009), interspecific interactions (Rota et al., 2016), animal behavior (Caravaggi et al., 2017; Rowcliffe, 2017; Caravaggi et al., 2020), and nest predation (Bayne and Hobson, 1997; Beck and Terborgh, 2002; Vilardell et al., 2012).
1.1.1. Documenting species presence
Documenting species presence or absence is crucial to the discovery (Rovero et al., 2008), rediscovery (Yamada et al., 2010), and confirmation (Lhota et al., 2012) of range expansions of both native (Chynoweth et al., 2015) and invasive (Naderi et al., 2020) species. Studies of species presence are pertinent to monitoring elusive and endangered species, and photos of these species are also invaluable for education and public outreach. Effects of human activity on species and ecosystem dynamics in remote and rural areas (Muhly et al., 2011; Gallo et al., 2017; Parsons et al., 2018) and conservation threats, such as the impact of poachers on wildlife populations, can also be monitored (Jenks et al., 2012).
Photographic evidence often renders species presence indisputable. However, photos of animals can be misinterpreted or indecipherable, leading to spurious claims of new species (Meijaard et al., 2006). These claims, along with apparent range expansions, rediscoveries, and retractions, may be a result of the lack of baseline information and insufficient density of camera traps (Dobson and Nowak, 2010). Researchers must acknowledge that non-detection is not the same as absence, as individual species' detection probabilities, which are almost always <1 (i.e., not all species in the area will be perfectly detected), may result in a species that is actually present within a study area going undetected during sampling (Tilson et al., 2004; MacKenzie et al., 2017).
Though several established methods effectively document species presence, comparison studies suggest that camera traps have higher probabilities than hair tunnels (O'Connell et al., 2006; Paull et al., 2012), cubby boxes (O'Connell et al., 2006), patrol observations (Burton, 2012), and line-transect surveys (Trolle et al., 2008) for detecting smaller, solitary, and nocturnal species (Wearn and Glover-Kapfer, 2019). Studies incorporating track plates used to document the presence of animals by recording footprints have shown that species richness and recording rates correlate with camera trapping results (Espartosa et al., 2011). In some cases, track plates were more effective (Hackett et al., 2007) and detected more individuals (Rosas-Rosas and Bender, 2012). Yet, with technological advances, remote cameras require less maintenance and may be more cost effective than track plates for studies >1 year (Ford et al., 2009). An alternative method for detecting presence is genotyping by scat collection, which has produced consistent (Galaverni et al., 2011) and sometimes better (Harrison et al., 2002) detection probabilities than camera traps. Finally, under proper weather conditions, snow tracking surveys have the highest probability of detection for species active in the winter (Gompper et al., 2006).
Study design for documenting species presence does not necessarily need to be systematic and can be targeted at specific sites or use species-specific baits to maximize detection probability. Furthermore, there is no minimum for the number of cameras a researcher should deploy for this type of analysis, but having more cameras increases detection probability and allows one to investigate across multiple different habitats, human influence levels, and other variables. Increasing the number of camera traps also decreases the amount of time the study will need to stay active in order to sample the species of interest. With species presence data, all that is needed is the photographs from the camera traps and the GPS locations of the cameras themselves. This is the simplest form of camera trap data used for research purposes.
1.1.2. Relative Abundance Index (RAI)
Camera trap data can be used to generate a Relative Abundance Index (RAI), which is typically calculated by summing detections (usually in the form of the number of “independent” photographs, where independence is denoted as a set amount of time that needs to pass before a photograph is deemed a new detection) for each species, dividing by the total number of active camera days, and multiplying this fraction by 100 (O'Brien, 2011). This approach is attractive to conservationists because of its simplicity. However, it has been criticized for being an inappropriate and unreliable method (Sollmann et al., 2013a). This index is known to produce biased estimates based on heterogeneous detection probabilities (Jennelle et al., 2002; Sollmann et al., 2013a), and as a result, its use needs to be justified as the only reasonable alternative to other methods (O'Brien, 2011).
The application of the RAI relies on the assumption that the index is directly related to true species abundance (O'Brien et al., 2003). The majority of RAI studies aim to estimate abundance at a single point in time at a specific site (e.g., protected area), but this index has also been used as an abundance proxy to study a variety of ecological processes including habitat use (Bowkett et al., 2008), human impacts on wildlife (Kinnaird and O'Brien, 2012), temporal population dynamics (Jenks et al., 2011), and activity patterns (Ramesh et al., 2012).
As discussed above, the main issue with RAI is that of detectability. Detectability varies among and within species and is considered a major source of bias (Larrucea et al., 2007; MacKenzie et al., 2017). Variations in detection probability due to species differences in behavior, life history characteristics, natural rarity, home range size, and temporal activity patterns have all been shown to bias RAI estimates (Sollmann et al., 2013a). With independently derived abundance estimates in a double sampling design, RAIs can be calibrated for a particular system or study area (O'Brien et al., 2003), but this also requires continuous re-calibration and results do not translate outside that area.
Study design for RAI surveys should aim to limit the effect of variation in detection probabilities to account for the main deficiency of this approach (Sollmann et al., 2013a). Once the study area is determined, cameras should be placed at distances smaller than the home range diameter of the target species to prevent false negatives. The number of cameras necessary depends on study area extent and target species, but should cover the area uniformly and at a great enough density to maximize detection probability. Furthermore, feature-focused designs (study designs focusing on trails, roads, streams, and other habitat features to increase the rate of detection) should proceed with extreme caution because of the inability to account for the differences in detectability across these features. Instead, studies using RAIs should consider setting up cameras randomly across the study area, where individual camera sites are most representative of the surrounding habitat. To calculate RAI or trap rate, only species presence data and trapping effort (the number of active camera days) are needed. If covariates of habitat structure, human influence, and other site variables are to be included in analysis, the GPS coordinates of each camera will also be needed. Methods described in the previous section can be used in lieu of camera traps to calculate RAIs (Jhala et al., 2011), but these methods may require more extensive fieldwork and physical trapping effort than camera traps.
1.1.3. Density estimates and individual recognition
Density estimates are a common objective of camera trapping studies and may be the most sought-after population parameter (O'Connell et al., 2011; Burton et al., 2015). Density estimates allow for easy comparisons between sites and years or extrapolation to larger areas (Bellan et al., 2013). If individuals can be identified in a population, capture-recapture methods can produce reliable estimates for a study area. Three main types of capture-recapture population models are used to estimate abundance: (i) closed—no birth, death, immigration or emigration (O'Brien, 2011), (ii) open—losses and recruitments are allowed (Gutiérrez-González et al., 2012), and (iii) spatially explicit—including spatial characteristics such as home range and individual mobility (Gardner et al., 2010; Royle et al., 2014; Royle et al., 2018; Green et al., 2020).
The sampling area for density estimate studies is typically set up in a grid-like system, with the outermost trap locations representing the study area boundary. To estimate the effective sampling area, the simplest approach is to draw a concave polygon by connecting the outermost trap locations in a geographic information system. However, this fails to include ingress from outside animals and outward movement from animals inside the polygon. A more appropriate approach is to estimate a buffer around this polygon. Though no consensus exists on calculating this area, a buffer of mean maximum distance moved (MMDM) of the target species is common. MMDM can be estimated from camera trap data, spatially explicit capture-recapture (SECR) models, or estimates based on auxiliary telemetry data. The MMDM method may inflate density estimates (Soisalo and Cavalcanti, 2006), which has led to the arbitrary but frequently used ½MMDM approach. Neither has a theoretical basis (Obbard et al., 2010). Auxiliary telemetry data, typically available from other studies on target species, is most effective at estimating MMDM (Dillon and Kelly, 2008; Núñez-Pérez, 2011).
Early in camera trapping science, two landmark papers estimated density of tigers by identifying individuals with unique pelage characteristics (Karanth, 1995; Karanth and Nichols, 1998). This approach has been extended to a variety of species to identify individuals based on spots (Jackson et al., 2006), stripes (Singh et al., 2010), muzzle markings (Mazzolli, 2010) and other forms of unique pelage (Caruso et al., 2012). Additionally, capture-recapture methods are possible if animals are captured and tagged with artificial markings such as ear tags or GPS collars (Jordan et al., 2011; Weckel and Rockwell, 2013). However, individual identification can be subject to researcher bias (Oliveira-Santos et al., 2010), and efforts have been made to incorporate a more rigorous Bayesian approach to individual identification (Stafford and Lloyd, 2011). Bilateral photo identification records from single trap stations can introduce inconsistencies due to bilateral asymmetry in coat patterns, but modeling approaches to combine left- and right-sided photos are being developed to address this (McClintock et al., 2013). Currently, a common and simple solution is to modify study design to include two cameras at each station (Negrões et al., 2012). Furthermore, study design issues related to sampling area, camera spacing, and detection probability may introduce significant biases (Dillon and Kelly, 2007; Foster and Harmsen, 2012), and recent literature on study design should be consulted before project implementation (Royle et al., 2018; Efford and Boulanger, 2019; Green et al., 2020).
Several reviews have focused on analysis techniques (Sharma et al., 2010; Obbard et al., 2010; Foster and Harmsen, 2012; Royle et al., 2018; Green et al., 2020), improving current capture-recapture analysis (Royle et al., 2009), and developing new techniques including Bayesian inferences for arbitrary sample sizes (Gardner et al., 2010) and maximum likelihood approaches (O'Brien and Kinnaird, 2011; Efford et al., 2019). In particular, SECR models use a hierarchical approach to model both detection probability and home range location and have produced more accurate density measurements in most studies (Kalle et al., 2011; Blanc et al., 2013; Royle et al., 2014; Royle et al., 2018; Green et al., 2020). Currently, these advances in density estimators have been used for relatively few species that can be individually identified by coat patterns (Green et al., 2020). Techniques to estimate density without individual identification have been proposed (Carbone et al., 2001; Rowcliffe et al., 2008; Manzo et al., 2012; Chandler and Royle, 2013), but have not been without criticisms (Foster and Harmsen, 2012). Finally, spatial mark-resight models require only partially marked populations, extending the number of species whose density can be estimated through camera trapping to species only partially individually identifiable and have garnered much attention in recent years (Sollmann et al., 2013b; Jimenez et al., 2017; Whittington et al., 2018).
1.1.4. Occupancy analysis
Reliable density estimates require rigorous study design and large quantities of resources. An alternative approach is occupancy modeling, an established method to model the probability of a site being occupied by a species (MacKenzie et al., 2002; O'Connell and Bailey, 2011; MacKenzie et al., 2017). Occupancy uses presence/absence (or, more appropriately, detection/non-detection) data from independent replicate surveys under the assumption that the population is closed during the survey period. Results provide estimates on the proportion of area occupied by a species. Conversely, if temporal or spatial closure is violated, the parameter estimated becomes the proportion of study sites used by a species. In addition, surveys can be conducted over time and space to elucidate how habitat covariates impact species occurrence. A major advantage of occupancy modeling is that it explicitly estimates and models detection probability (Jones, 2011; MacKenzie et al., 2017). Generally, there is a positive relationship between occupancy and abundance, and occupancy has been used as a proxy for abundance in studies of niche partitioning (Di Bitetti et al., 2010), impact of human disturbance (Mohamed et al., 2013), and predator-prey dynamics (Silva-Rodríguez and Sieving, 2012). However, when spatial and/or temporal closure is violated, occupancy should not be used as a proxy for abundance, as species with varying home ranges and densities will produce biases in multi-species estimates. Typically, occupancy requires smaller sample sizes and is therefore typically less expensive and time-consuming than density estimation (MacKenzie et al., 2017). A rich literature exists on modeling species occupancy, and a wide variety of presence/absence (detection/non-detection) data (Vojta, 2005; MacKenzie et al., 2006) have been used in a number of camera trap studies (Erb et al., 2012; Gopalaswamy et al., 2012a; Schuette et al., 2013; Burton et al., 2015; MacKenzie et al., 2017).
Study design for occupancy models requires a camera array that provides a representative sample of the study area, or a sample design where habitat heterogeneity is incorporated into analysis covariates. Occupancy models allow for stations to be shifted between units, given that they are present at each location long enough to collect sufficient data (O'Connell and Bailey, 2011; MacKenzie et al., 2017). Cameras should be spaced at a distance greater than the minimum of the diameter of the target species' home range, unless the goal of the study is to estimate habitat use instead of the proportion of area occupied. Information on environmental conditions for each site also need to be collected if researchers choose to include habitat covariates in their occupancy model. Finally, prior work has focused on camera trap design for occupancy studies, and we direct readers to these for more detailed information on study design criteria such as the number of sites, spatial replicates, and others design parameters (MacKenzie and Royle, 2005; Guillera-Arroita et al., 2010; Whittington et al., 2018; Kays et al., 2020).
1.1.5. Activity analysis
Camera trap data can be used to elucidate diel and seasonal activity patterns and understand interspecific competition and niche partitioning (Linkie and Ridout, 2011; Rota et al., 2016; Parsons et al., 2016; Frey et al., 2017). Camera traps allow researchers to record multiple species over long periods with minimal disturbance (Ramesh and Kalle, 2013). Much work has been done with sympatric species, such as felids and canids (Foster et al., 2013; Athreya et al., 2013) and on observations of predator-prey dynamics (Weckel et al., 2006; Ford and Clevenger, 2010; Linkie and Ridout, 2011). Especially important for conservation, human impact on animal activity, including human-wildlife coexistence, has also been investigated (Carter et al., 2012; Wang et al., 2015; Gaynor et al., 2018). However, co-occurrence does not necessarily equal coexistence (Harihar et al., 2013), and camera trapping data may fail to capture important factors that determine species activity and distribution. Specifically, camera traps can tell researchers where an individual animal is or has been, but it cannot tell them how that individual got there.
Though camera traps enable researchers to gain new insights into the activity patterns of wild animals, pairing cameras with other approaches can produce more reliable data. Most telemetry collars are now equipped with an activity sensor that uses triaxial accelerometers that record movement of an animal's neck at very high temporal resolutions. Combined with movement data from GPS location and camera trap data, detailed animal activity can be observed. More recently, National Geographic Crittercams© and BBC's animal cameras have been deployed on large mammal species to document previously unknown activity and behavior (Şekercioğlu, 2013; PBS, 2018).
Study design for activity surveys focuses on documenting temporal and seasonal presence data and therefore should strive to maximize detection probabilities for target species. Camera placement on game trails and other areas frequented by animals may increase captures, but may also produce bias in activity estimates if species concentrate on certain features during specific times of the day. To avoid biases associated with detection, study design must aim to have equal detection probabilities between species or account for these presumed differences with both species-specific and site-specific covariates. Recent reviews on activity and behavior analysis should be consulted for further information on study design recommendations and potential sources of bias when designing camera trap studies for activity analysis (Caravaggi et al., 2017; Frey et al., 2017).
2. Methods
2.1. Literature review
We conducted a systematic search of peer-reviewed literature published between 1975 and 2020 using search terms related to camera traps and animal ecology (Table 1 ). Every combination of these terms was used in a search of the ISI Web of Knowledge Complete Collection database search engine. Each article was reviewed to confirm that it discussed camera traps. The database included: publication year, article title, journal name, target taxa, study country, paper type, and primary objective. Finally, to assess the recent extent to which camera traps have been used for urban ecology research, we conducted a keyword search of abstracts from papers published after 1 January 2018 referencing either urban or suburban study sites.
Table 1.
Camera trap and animal ecology keywords used in literature search of the ISI Web of Knowledge.
| Camera trap terms | Animal ecology terms |
|---|---|
| “Camera Trap*” | Wildlife |
| “Game Camera*” | Birds |
| “Trail Camera*” | Mammals |
| “Remote Photography” | Reptiles |
| Amphibians |
3. Results
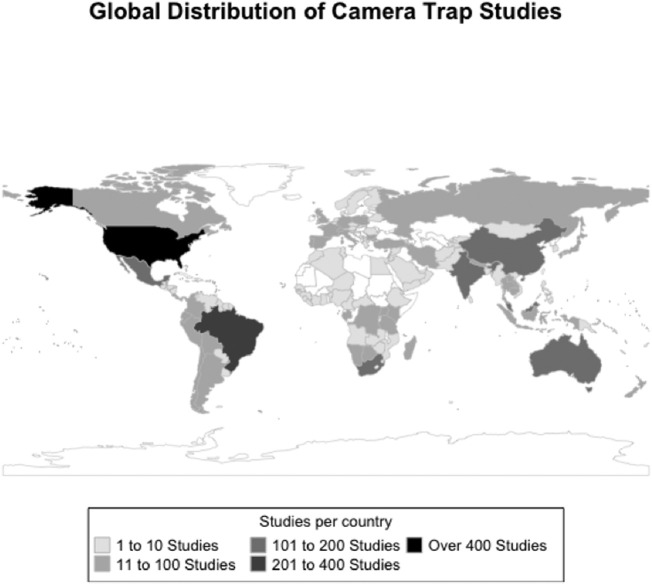
Our review of camera trapping studies from 1975 to 2020 (Supplementary Table 1) reveals that publications have increased at a rapid rate, with over 500 articles published in 2020 alone (Fig. 1). In the ISI Web of Knowledge Complete Collection, there were no camera trapping papers published prior to 1993. Our literature search resulted in 3326 papers published across 433 journals, with 258 journals having more than two articles and 75 journals having >10 articles (see top 10 journals in Table 2). Research was conducted in 124 countries (Fig. 2). Target taxa included mammals, birds, herpetofauna, and multiple other taxa (Table 3). The majority of articles covered studies of mammals (85.9%), most of which belonged to the order Carnivora (45.9%), the majority of which were felids (52.4%). A considerable number of studies focused on multiple species, with 26.8% of mammal studies including species from multiple taxa (22.2% of total dataset). This is an underestimate, however, given the presence of multi-species studies focusing entirely within the same order (e.g., 17.7% of carnivore studies included multiple families, Supplementary Table 1). The primary objective of most studies was investigating different methodology (11.9%). Papers also had primary objectives of estimating density (11.7%), species presence (9.5%), relative abundance (9.1%), occupancy (8.8%), activity (7.6%), and species richness (5.1%). Most recent studies were conducted in protected or non-urban areas, as a keyword search of all abstracts for papers published since 2018 (n = 1375) shows that only 1.1% (n = 15 papers) targeted urban or suburban environments.
Fig. 1.

Camera trapping studies by year published from 1990 to 2020 based on a systematic search of key terms in ISI Web of Knowledge.
Table 2.
Number of camera trapping articles published in the top ten journals from 1990 to 2020 based on a systematic search of key terms in ISI Web of Knowledge.
| Journal | Number of articles |
|---|---|
| PLoS One | 157 |
| Oryx | 136 |
| Biological Conservation | 115 |
| Wildlife Research | 86 |
| Journal of Mammalogy | 84 |
| European Journal of Wildlife Research | 78 |
| Mammalia | 73 |
| Wildlife Society Bulletin | 68 |
| Mammalian Biology | 67 |
| Journal of Wildlife Management | 60 |
Fig. 2.
The global distribution of camera trapping studies published from 1990 to 2020 based on a systematic search of key terms in ISI Web of Knowledge.
Table 3.
Proportion of target taxa in camera trapping studies published from 1990 to 2020 based on a systematic search of key terms in ISI Web of Knowledge.
| Taxa | Percent of total articlesa |
|---|---|
| Mammal | 82.9 |
| Bird | 5.9 |
| Multiple taxa | 5.7 |
| Herpetofauna | 1.7 |
| Insect | 0.2 |
| Within mammal order diversity | Percent of mammal category |
|---|---|
| Carnivore | 45.9 |
| Multiple Orders | 26.8 |
| Ungulate | 13.2 |
| Rodent | 5.5 |
| Primate | 4.3 |
| Marsupial | 1.8 |
| Other | 2.3 |
| Within carnivore family diversity | Percent of carnivore category |
|---|---|
| Felidae | 52.4 |
| Multiple Families | 17.7 |
| Canidae | 11.8 |
| Ursidae | 7.1 |
| Mustelidae | 6.8 |
| Hyaenidae | 1.3 |
| Other | 2.9 |
Percent of total articles does not add up to 100% (96.4%) because review articles were present in the database.
4. Discussion
4.1. Importance of camera trapping for conservation research and community engagement
In the past decade, much work has been done to improve the scientific rigor of camera trapping studies. Camera trapping science is evolving rapidly, and scientists and practitioners emphasize that carefully executed study designs can yield informative parameters, as described in the sections above. However, the potential of simple, inexpensive camera deployments to revolutionize conservation projects with budgetary restrictions should also be recognized. It has been suggested that there are two categories of camera trap studies: (1) science, understanding how an ecosystem works, and (2) management, moving an ecosystem from less to more desirable states (Nichols et al., 2011). We assert that conservation outreach, community science, and environmental education constitute a third category (Adler et al., 2020). While other experts have suggested that photos are the means to an end goal of informing the larger process of science and management (Nichols et al., 2011), we also affirm the value of photographic records of elusive species. For example, the authors' existing conservation project in eastern Turkey initially deployed four camera traps at a study site in 2006. The documentation of an unexpectedly high relative abundance of large carnivores and the scarcity of their prey species has led to national and international support for a large-scale monitoring project for mammals and catalyzed the government to designate Turkey's first wildlife corridor. A conservation success in a country experiencing a major biodiversity crisis (Şekercioğlu et al., 2011), the project has since evolved into a more rigorous study with a network of 40 camera traps being systematically deployed over a multi-year period.
Camera trap photos and videos are also effective public outreach tools that raise awareness about important study sites, vulnerable species, and conservation priorities of local and global organizations or governmental agencies. A single photo published via social and traditional media can deliver important conservation messages to millions of people. The authors share camera trap photos and project updates on Facebook and Instagram, where a single photo can be viewed by over 25,000 individuals in a five-day period. Public outreach opportunities extend to citizen science approaches (Adler et al., 2020) in which members of the public deploy cameras or identify species in camera trap photos. Several large-scale camera trapping efforts, such as the Tropical Ecology Assessment and Monitoring Network (TEAM; www.teamnetwork.org; also see Fegraus et al., 2011) Smithsonian Wild (see http://siwild.si.edu), eMammal (https://emammal.si.edu), Wildlife Insights (https://www.wildlifeinsights.org/home), EUROMAMMALS (https://euromammals.org), and the Urban Wildlife Information Network (https://urbanwildlifeinfo.org) have already made progress through citizen science and multi-city collaboration efforts.
4.1.1. Guiding questions during COVID-19
The effects of COVID-19 lockdowns will most likely have a marked impact on where animals go and what habitats they access. Simple comparison studies on species presence before, during, and after a marked change in human activity can help scientists understand the effects of human influence on wildlife distribution. Camera traps can help identify which species' ranges extend, contract, or stay the same in the face of changing human influence and global decreases in human travel. Anecdotal evidence of wildlife reclaiming cities during lockdown may be a sign of species range extensions (Sahagun, 2020). However, as Zellmer et al. (2020) point out, this may also be a result of people using time previously spent commuting or gathering socially to observe the wildlife that have always been present in their cities (Garrard et al., 2008). Furthermore, some locales are actually seeing great increases in recreational traffic on public lands and urban greenspaces, as people seek out leisure activities that adhere to the CDC's current social distancing guidelines (Samuelsson et al., 2020; Slater et al., 2020; Razani et al., 2020; Rice et al., 2020). Camera traps have already been used to observe urban species often overlooked by the public (Magle et al., 2019), and future camera trap studies could contribute to the growing literature of new species accounts in urban areas (Feinberg et al., 2014; Hartop et al., 2015). Therefore, empirical investigations of species either reclaiming urban habitat during COVID-19 shutdowns or retreating from habitat in areas experiencing increases in recreational traffic are needed before anecdotal observations can be substantiated. Furthermore, these changes in species distribution, colonization, and retreat can be modeled across geographies, identifying climatic, socioeconomic, and environmental factors that may be influencing these changes. These baseline investigations will provide scientists with a better mechanistic understanding of how human traffic effects the wildlife use of habitat within a wildland-urban interface (Martinuzzi et al., 2015).
One challenge for researchers will be to document and quantify the variety of impacts COVID-19 restrictions have had on human activity and behavior in order to understand how wildlife communities respond. The results from our review demonstrate the geographic disparity that exists regarding where camera trap studies occur (Fig. 2). This is unsurprising, as many research fields follow similar patterns. For researchers in countries with high numbers of camera trap studies (e.g., USA), opportunities already exist to collaborate or conduct meta-analyses (see below), with datasets across a variety of pandemic-caused lockdown scenarios. This will make it easier to detect the effect of this anthropause on wildlife. Our review also highlights regions of the world where camera trap publications are lacking or lagging, and opportunities may exist to encourage camera trap use or peer-reviewed publication of results in these areas.
Documenting changes in species presence across a landscape can only answer questions on how species distributions may change in response to changes in human mobility and traffic. However, it cannot discern the extent to which species use a particular habitat. Using camera traps to calculate species-specific, community-specific, and study-site specific RAIs as a relative measure of habitat preference, scientists can go beyond investigations of species presence, range expansions, and range contractions and examine the scope of this change. Are species, communities, and populations colonizing new areas in relatively large numbers, or are these colonization events restricted to vagrant individuals or small groups? RAI can also be used for measuring the differences in species temporal activity and species-species interactions. Specifically, investigating questions concerning how individual species change their activity and behavior in response to the COVID-19 pandemic can help scientists understand the extent that species have adapted to living in urban and semi-urban areas, as well as identify novel changes in behavior in response to an unprecedented change in human influence. As mentioned above, other methods exist to calculate RAI, but none are as well-suited during current field research restrictions as are camera traps. However, it is important to note that using RAI to investigate changes in habitat use, relative abundance, and behavior should be done with caution, and investigators should pay close attention to the concerns noted above.
Density is a state variable of conservation concern, and is often considered the pinnacle of biodiversity assessment (Williams et al., 2002; Royle et al., 2014). With density estimates, scientists and wildlife managers have the most accurate measure of population assessment available. During COVID-19, quantifying potential changes in density is one of the best ways to assess how wildlife react to human influence. Long-term studies continuing their work through and after the pandemic will be in a position to measure how specific species react to changes in human activity in response to the pandemic, including increases in hiking and recreational traffic, vehicle traffic, CO2 emissions, air pollution, anthropogenic food resources, and other impacts specific to the effects of lockdowns. Camera traps are valuable tools in measuring density of both fully marked and partially marked species and do not require direct capture, substantially lowering the amount of work required in the field. For this reason, it is important for researchers interested in collecting density estimates to consider study design and study species carefully, as only a subset of the wildlife community will be available for sampling, and density estimation requires large amounts of data from potentially rare and elusive species (Royle et al., 2018; Green et al., 2020).
Occupancy modeling is one of the fastest growing analysis methodologies in camera trapping. Like much of the methodology described above, occupancy modeling is particularly powerful when researchers are interested in distributions, species-species interactions, and habitat use dynamics and preferences. A strength of occupancy modeling is that the analysis directly accounts for detection error and allows for both detection probability and occupancy to vary in response to site-level and species-specific covariates, as opposed to simple presence/absence surveys and RAIs. Occupancy modeling is less-intensive and does not require as many resources as density surveys; however, it also lacks the capacity to directly monitor population size through time. Since the analysis does not require individual recognition, it is ideal for surveys looking at multiple species (Rich et al., 2016). This feature makes occupancy modeling an exciting avenue for investigating how species-species interactions may change in response to the effects of COVID-19 lockdowns, as well as how entire communities may be changing as a result of the global change in human mobility.
One of the most pressing ecological questions during the COVID-19 pandemic is the effect the sudden change in human impact is having on wildlife behavior. Camera traps are perfectly suited to capture these changes, especially in regards to temporal activity and spatiotemporal behavior (Frey et al., 2017). Temporal activity changes have already been noted during periods of increase in human activity (Gaynor et al., 2018), with animals shifting their activity to become more nocturnal. Investigating if the reverse trend holds during decreases in human traffic is of paramount importance during this period of global decrease in human mobility. Furthermore, utilizing the variation in shutdown intensity across municipalities will illuminate whether these changes are species-specific, vary across climates and socioeconomic status, and are linear or threshold-based. Finally, camera traps can be used to investigate how species' spatiotemporal activity changes in response to biotic and abiotic factors (Parsons et al., 2016; Naidoo and Burton, 2020). From this information, it is possible to elucidate whether species are attracted to or avoid one another (Parsons et al., 2016), providing valuable insight on predator-prey dynamics and interspecies interactions. This information could be used to investigate how species are using space and time in relation to human influence (Ditmer et al., 2020; Fidino et al.,2020; Naidoo and Burton, 2020) and how this relationship has changed in the wake of COVID-19.
As explained above, camera traps have been used extensively to understand the large-scale spatial distribution of species. However, camera traps can also be used for studying the fine-scale behavior of species (Caravaggi et al., 2017), including social interactions (Leuchtenberger et al., 2014) and anti-predator behavior (Carthey and Banks, 2016). Most modern camera traps come with a video setting, making it possible to capture short video clips each time the camera is triggered. This technology allows researchers to establish experimental and quasi-experimental systems to elucidate how wildlife react to different environmental stimuli. To date, a lot of work has been done to study wildlife behavior in natural settings (Caravaggi et al., 2017), but less work has been done on elucidating the fine-scale effects of human influence on wildlife behavior (but see Gallo et al., 2019; Breck et al., 2019). We see this as one of the most fascinating and hereto underutilized opportunities for camera trap research in the future, especially during this era of rapid global change. However, detector-caused bias must be considered when using camera traps for fine-scale behavioral research (Caravaggi et al., 2020), and practitioners should consider the possibility that the cameras themselves may elicit a response (i.e., it is necessary to establish an experimental control to test the behaviroal effect cameras may have on wildlife). Furthermore, using camera traps to gather videos can drain batteries and fill up memory cards faster than using them for photos, which may result in the need for more intensive fieldwork. This may not be possible in many areas experiencing restrictions in fieldwork activity.
Finally, although COVID-19 has had a marked effect on conservation, research, tourism, and ecotourism activities (Bakar and Rosbi, 2020; Buckley, 2020), COVID-19 movement bans do not seem to have stopped illegal activity, and in some cases, there is evidence that such activity has increased (McNamara et al., 2020; Buckley, 2020). Banning researchers, ecotourists, wildlife managers, rangers, and government personnel from going out into the field results in fewer people to notice and report poachers, illegal loggers, collectors, and developers. This further highlights the importance of camera traps as conservation tools. GPS/GSM camera traps can email photos to law enforcement officials upon taking them, facilitating the identification of poachers and triggering an immediate enforcement response. For example, in 2019, Panthera updated and released their newest version of the PoacherCam (Panthera, New York City, USA), which features a small, easily-hidden, motion-activated trail camera with the capacity to take images in the field, process images for photos of humans using built-in Artificial Intelligence technology, and wirelessly send human photos to authorities. The technology allows officials to setup cameras outside or near the peripheries of protected areas and use them to catch poachers before they are able to make a kill. Other technology and camera trap companies have followed suit (see https://www.resolve.ngo/trailguard.htm), which will make these highly-specialized cameras more affordable for deployment anywhere in the world.
A final set of guiding questions focuses on the target taxa of most camera trapping studies. Our review highlights the overwhelming focus of camera trap studies on mammals (83.2%), specifically carnivores (46.0% of mammal category). Within the carnivore category, over half of the published papers focused on felids. This indicates that more data may exist for species that are particularly sensitive to human activity and environmental change (Ripple et al., 2014). These species could serve as indicator species for wildlife response to our current anthropause. Finally, our results highlight the lack of target species in other taxa, suggesting we may be able to apply camera trapping as a method to a broader range of taxa during periods of field research shutdowns.
4.2. Future of camera trapping in conservation biology and ecology: during and beyond COVID-19
In the past two decades, camera trapping has emerged as an important method for conservation biology and ecology research, and the rapid increase in studies using these tools is likely to continue. Research questions on presence/absence and basic ecology of animals are valuable to conservation efforts. However, further development of study designs, analyses, and standardization of reporting camera trap results is needed (Meek et al., 2014; Steenweg et al., 2017). Currently, few studies go beyond baseline assessments (Linkie et al., 2010), but as the price of equipment decreases, broad scale landscape ecology studies can incorporate camera traps to address novel questions in conservation biology (Erb et al., 2012; Rich et al., 2016, Rich et al., 2017).
Our literature review highlights the benefits of camera traps as a low cost, low maintenance, and largely non-invasive monitoring tool for conservation biology research and applied conservation projects. Many studies in our review documented understudied species in remote areas and significant camera trapping findings contributed to the conservation of species and ecosystems. Using the growing body of literature, conservationists can ensure they are defining questions a priori and making inferences using appropriate analyses and statistical techniques.
As more sophisticated studies are designed, camera traps will help shape large-scale conservation agendas, especially across protected areas (Kinnaird and O'Brien, 2012; Li et al., 2012). Camera trapping has been increasingly discussed as a method for conservation hotspot analyses (Kouakou et al., 2011), monitoring biodiversity (Waldon et al., 2011; Steenweg et al., 2017), comparing human dominated landscapes to natural areas (Cassano et al., 2012; Gallo et al., 2017; Parsons et al., 2018; Parsons et al., 2019), and assessing how animals respond to fluctuations in human activity (Harihar et al., 2009; Mohamed et al., 2013; Gallo et al., 2017). Camera traps have already helped biologists document unexpected wildlife presence in human-dominated landscapes (Athreya et al., 2013). If global camera trapping efforts can be standardized (Ahumada et al., 2011) and coordinated, camera traps could contribute to a comprehensive global mammal conservation strategy (Rondinini et al., 2011). However, our review highlights important geographic gaps in where camera trap studies are occurring. Furthermore, if data management issues are addressed, meta-analyses of current data could be pursued for regional analysis of abundance and diversity (Ordeñana et al., 2010).
In the unprecedented era of COVID-19, conservation biology and ecology research has been hampered by rigid restrictions on travel and field research. The resulting landscape is forcing researchers the world over to develop creative new ways of investigating our natural world (Maas et al., 2020). In this arena, camera traps offer a path forward. Camera traps are cost-effective, non-invasive survey tools that require little maintenance in the field and can be left monitoring for long periods of time. Furthermore, due to ongoing CDC social distancing guidelines and local government restrictions on mobility, researchers are experiencing at least periodic bans on fieldwork. For example, the authors lost an entire field season of live-trapping and bird banding in Utah due to a university-wide field research ban, but our active camera trapping projects continued.
With improving battery life and higher capacity memory cards, camera traps deployed in the field do not require extensive maintenance and can be left alone to monitor for long periods of time. Certain models are capable of being deployed in the field for up to a year with the ability to wirelessly send photographs without manual checks. Because camera traps can continue to collect critical wildlife data despite travel and research restrictions, they are currently more crucial to scientific research than ever before. Researchers can plan for potential restrictions by changing the settings on cameras to collect fewer images per trigger or program cameras to be active during certain times in order to prolong battery life and data storage capacity. If researchers carefully determine study objectives, the data collection (i.e., camera settings) can be selected to maximize how long cameras can remain in the field without service. As previously mentioned, one potential limitation would be studies that require video recording, typically for study objectives focused on animal behavior.
This, however, poses a very important question: how does one start a camera trapping project during the pandemic, and are there active projects that are looking for additional collaborators? First, the research team must clearly articulate the question they hope to address and decide if camera trapping is a valid tool for their research (i.e., can camera traps help answer the particular question of interest?). If so, the team must then decide on a proper study design and camera model. This will vary based on target species, research goals, study area, and other parameters, but spending more time on design and implementation beforehand will pay big dividends in the long-run (Rovero et al., 2013; Kays et al., 2020). Specifically, running simulation and power analysis using readily available software may aid researchers in avoiding a sub-optimal design (Gerrodette, 1987; Steenweg et al., 2016; Efford and Boulanger, 2019), especially if the goal is to monitor trends through space or time (Green et al., 2020). We outline only the basics of camera trap study design in the above sections, and we strongly encourage new researchers to consult earlier work on this topic (MacKenzie and Royle, 2005; Guillera-Arroita et al., 2010; Frey et al., 2017; Green et al., 2020; Kays et al., 2020). Furthermore, there are multiple research groups that have continued work during the COVID-19 pandemic, with existing datasets going back years before COVID-19. Here, we highlight a few of the larger collaborations and research groups. Each of these groups has continued their work during the pandemic, and they plan to continue doing so long after the pandemic has passed. We hope that by highlighting these organizations, we encourage conservation researchers throughout the globe to harness the power of camera traps as a research tool, take advantage of the already existing infrastructure to investigate conservation questions at a large scale, and leverage the power of collaboration and rigorous study design to advance camera trapping to the forefront of ecological research.
The Urban Wildlife Information Network (UWIN; Magle et al., 2019), led by the Lincoln Park Zoo's Urban Wildlife Institute (https://urbanwildlifeinfo.org), is a partnering organization of researchers that use camera traps and other non-invasive monitoring techniques to study the ecology and behavior of urban wildlife. With partnering institutions across all of North America, UWIN is a good collaboration to study the effects of COVID-19 lockdown on urban-adapted species. Furthermore, UWIN is actively looking for research groups to join their team, especially groups outside of the United States and Canada.
eMammal (https://emammal.si.edu) is an online data management tool built specifically for camera trap research throughout the globe. The project offers resources for practitioners in the form of data entry, upload, and review tools; project page development; training for camera trappers and volunteers; study design and camera selection recommendations; and data formatting, analysis, and visualization pipelines. Furthermore, eMammal is the host of Snapshot USA (https://emammal.si.edu/snapshot-usa), a nationwide collaboration dedicated to examining trends in mammal communities across a gradient of human influence and climatic conditions (Cove et al., 2021). Snapshot USA was active for the 2020 field season, even under COVID-19 restrictions.
EUROMAMMALS (https://euromammals.org) is the umbrella project for multiple continent-wide, species-specific studies looking at understanding the movement ecology of European mammals across different habitats and anthropogenic influence levels. Although not initially a camera trapping initiative, the project has grown to incorporate camera trap technology into their protocol.
Finally, Wildlife Insights (https://www.wildlifeinsights.org) is another collaborative effort between conservation organizations, professional scientists, and Google (Ahumada et al., 2020). Although currently in the beta stages of development, Wildlife Insights looks to address four major barriers to most camera trap practitioners, including data entry, data sharing, data analysis, and hardware issues, by leveraging an easy-to-use online interface and project builder with the Artificial Intelligence capabilities of big technology companies.
4.2.1. How current camera trapping efforts can inform future work
Our review highlights multiple aspects of camera trap research that may be utilized by future investigators, especially during lockdown. First, much of the current camera trapping research has originated in the Americas, with fewer studies published in Asia and Austraila and even fewer published in Africa and Europe. This discrepancy offers a major opportunity for researchers in these less-studied areas, especially during the pandemic. Many of these understudied areas are home to both rapid economic and urban development (e.g., southeast Asia and southern Africa) and older, established urban centers (e.g., Europe), providing an opportunity to advance urban wildlife research with comparative, multi-city analyses. Assessing the differences in urban wildlife response across age and structure of development and the effects these factors have on wildlife distribution and behavior in response to lockdown are fascinating avenues for future research. Furthermore, the rapid growth of organized networks like the ones mentioned above provide both the framework and resources to conduct such studies at both larger and more localized scales.
Second, as mentioned previously, the breakdown of taxa studied hitherto highlights an important advantage of camera trap research, especially during a pandemic that has resulted in lockdowns across the globe. Most camera trap research is currently focused on rare, elusive, and carnivorous mammals (Table 3), species likely to be affected by changes in human activities. Carnivorous mammals are often considered both exceptionally important to natural ecosystems and human-intolerant (Ripple et al., 2014). These species can be difficult to study through other means, given their elusive behavior, natural rarity, and wariness of human beings. In fact, a recent review of density estimation research with camera traps found that for many of the mammalian carnivores studied, estimates of density represented the first and only ever reported for that species (Green et al., 2020). Furthermore, camera traps are increasingly used to study mammalian carnivores in urban areas (Gallo et al., 2017; Gallo et al., 2019; Breck et al., 2019), with research networks and collaborations around the globe dedicated to studying the effects of human influence on these species (e.g., UWIN and Snapshot USA). This means researchers can leverage both these large networks and camera traps to understand the effect lockdown has had on mammalian carnivore distribution and behavior, especially along urban, suburban, and exurban gradients, something that would be exceedingly difficult using other methodologies.
This review highlights three recurring takeaways for researchers using camera traps in the future. First, detection probability and survey effort are frequently ignored, but these elements are fundamental to the inferences that can be made from camera trapping data. Scientists, managers and conservationists should be careful when comparing or applying results from camera trapping studies that do not address these issues. Second, camera trapping may not be the most suitable method to address all given conservation biology questions, and although they are effective, they are not a panacea. Many reliable methods can document the presence of species and lead to accurate estimations of population parameters. Some of the most successful studies in our review used camera traps in conjunction with other techniques to generate estimates of target species density (Gopalaswamy et al., 2012b) and a more holistic picture of population dynamics (Palomares et al., 2012). Finally, camera traps, especially in the time of COVID-19, are invaluable, cost-effective, and relatively low-labor research and remote monitoring tools for conservation biology and ecology. They can be used to help answer questions on a broad range of topics. If used effectively, camera traps may be used to continue research on novel questions during lockdown that would otherwise be impossible.
Given the current benefits and future prospects of camera traps to address objectives in animal ecology and conservation biology, the continued surge in peer-reviewed publications over the last few years is encouraging. This surge is only expected to increase during the COVID-19 pandemic. Our literature review used only the Web of Science™ database and did not include gray literature from conservation organizations or government agencies. Inclusion would have increased our sample size of papers, but we believe that we would have reached similar conclusions. In light of the current popularity of camera traps, biologists must carefully define their questions and objectives before field data collection. With careful planning and study design, COVID-19 is likely to put camera traps at the forefront of ecology and conservation biology to help us further understand the impact of human activity on wildlife and generate solutions to promote human-wildlife coexistence.
The following is the supplementary data related to this article.
Full dataset of articles included in the systematic review of camera trap literature within the ISI Web of Knowledge Complete Collection.
Credit authorship contribution statement
Austin M. Green: Conceptualization, Methodology, Validation, Investigation, Data Curation, Writing – Original Draft, Supervision, Project Administration; J. David Blount: Methodology, Validation, Formal Analysis, Investigation, Data Curation, Writing – Original Draft, Visualization; Mark W. Chynoweth: Conceptualization, Methodology, Validation, Investigation, Data Curation, Writing – Original Draft, Visualization; Cagan H. Sekercioglu: Conceptualization, Methodology, Validation, Resources, Writing – Review & Editing, Supervision, Project Administration.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgements
This work was greatly improved by the comments and suggestions of three anonymous reviewers and the handling editor. AG would like to thank the Global Change and Sustainability Center, the Sustainable Campus Initiative Fund, the National Geogaphic Society, and the University of Utah Graduate Research Fellowship Program. Part of this work was also supported by the National Science Foundation Graduate Research Fellowship under Grant No. 2010094953 for MWC. DB and ÇHŞ thank Hamit Batubay Özkan and Barbara J. Watkins for their generous support, as well as the Conservation Ecology Graduate Fellowship and the Environmental Studies Graduate Fellowship. This research did not receive any other specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Adler F.R., Green A.M., Şekercioğlu Ç.H. Citizen science in ecology: a place for humans in nature. Ann. N. Y. Acad. Sci. 2020;1469:52–64. doi: 10.1111/nyas.14340. [DOI] [PubMed] [Google Scholar]
- Ahumada J.A., Silva C.E.F., Gajapersad K., Hallam C., Hurtado J., Martin E., McWilliam A., Mugerwa B., O'Brien T., Rovero F., Sheil D., Spironello W.R., Winarni N., Andelman S.J. Community structure and diversity of tropical forest mammals: data from a global camera trap network. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2011;366:2703–2711. doi: 10.1098/rstb.2011.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahumada J.A., Fegraus E., Birch T., Flores N., Kays R., O'Brien T.G., Palmer J., Schuttler S., Zhao J.Y., Jetz W., Kinnarid M., Kulkarni S., Lyet A., Thau D., Duong M., Oliver R., Dancer A. Wildlife insights: a platform to maximize the potential of camera trap and other passive sensor wildlife data for the planet. Environ. Conserv. 2020;47:1–6. [Google Scholar]
- Athreya V., Odden M., Linnell J.D.C., Krishnaswamy J., Karanth U. Big cats in our backyards: persistence of large carnivores in a human dominated landscape in India. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakar N.A., Rosbi S. Effect of coronavirus disease (COVID-19) to tourism industry. Int. J. Adv. Eng. Res. Sci. 2020;7 [Google Scholar]
- Bates A.E., Primack R.B., Moraga P., Duarte C.M. COVID-19 pandemic and associated lockdown as a “global human confinement experiement” to investigate biodiversity conservation. Biol. Conserv. 2020;248:108665. doi: 10.1016/j.biocon.2020.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne E.M., Hobson K.A. Comparing the effects of landscape fragmentation by forestry and agriculture on predation of artificial nests. Conserv. Biol. 1997;11:1418–1429. [Google Scholar]
- Beck H., Terborgh J. Groves versus isolates: how spatial aggregation of Astrocaryum murumuru palms affects seed removal. J. Trop. Ecol. 2002:18. [Google Scholar]
- Bellan S.E., Gimenez O., Choquet R., Getz W.M. A hierarchical distance sampling approach to estimating mortality rates from opportunistic carcass surveillance data. Methods Ecol. Evol. 2013;4:361–369. doi: 10.1111/2041-210x.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett N.J., Finkbeiner E.M., Ban N.C., Belhabib D., Jupiter S.D., Kittinger J.N., Mangubhai S., Scholtens J., Gill D., Christie P. The COVID-19 pandemic, small-scale fisheries and coastal fishing communities. Coast. Manag. 2020;48:336–347. [Google Scholar]
- Blanc L., Marboutin E., Gatti S., Gimenez O. Abundance of rare and elusive species: empirical investigation of closed versus spatially explicit capture-recapture models with lynx as a case study. J. Wildl. Manag. 2013;77:372–378. [Google Scholar]
- Bowkett A.E., Rovero F., Marshall A.R. The use of camera-trap data to model habitat use by antelope species in the Udzungwa Mountain forests, Tanzania. Afr. J. Ecol. 2008;46:479–487. [Google Scholar]
- Breck S.W., Poessel S.A., Mahoney P., Young J.K. The intrepid coyote: a comparison of bold and exploratory behavior in coyotes from urban and rural environments. Sci. Rep. 2019;9:2104. doi: 10.1038/s41598-019-38543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley R. Conservation implications of COVID-19: effects via tourism and extractive industries. Biol. Conserv. 2020;247:108640. doi: 10.1016/j.biocon.2020.108640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A.C. Critical evaluation of a long-term, locally-based wildlife monitoring program in West Africa. Biodivers. Conserv. 2012;21:3079–3094. [Google Scholar]
- Burton A.C., Neilson E., Moreira D., Ladle A., Steenweg R., Fisher J.T., Bayne E., Boutin S. REVIEW: wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J. Appl. Ecol. 2015;52:675–685. [Google Scholar]
- Caravaggi A., Banks P.B., Burton A.C., Finlay C.M.V., Haswell P.M., Hayward M.W., Rowcliffe M.J., Wood M.D. A review of camera trapping for conservation behaviour research. Remote Sens. Ecol. Conserv. 2017;3:109–122. [Google Scholar]
- Caravaggi A., Burton A.C., Clark D.A., Fisher J.T., Grass A., Green S., Hobaiter C., Hofmeester T.R., Kalan A.K., Rabaiotti D., Rivet D. A review of factors to consider when using camera traps to study animal behavior to inform wildlfie ecology and conservation. Conserv. Sci. Pract. 2020;2 [Google Scholar]
- Carbone C., Christie S., Conforti K., Coulson T., Franklin N., Ginsberg J.R., Griffiths M., Holden J., Kawanishi K., Kinnaird M., Laidlaw R., Lynam A., Macdonald D.W., Martyr D., McDougal C., Nath L., O'Brien T., Seidensticker J., Smith D.J.L., Sunquist M., Tilson R., Shahruddin W.N. The use of photographic rates to estimate densities of tigers and other cryptic mammals. Anim. Conserv. 2001;4:75–79. [Google Scholar]
- Carter N.H., Shrestha B.K., Karki J.B., Pradhan N.M.B., Liu J. Coexistence between wildlife and humans at fine spatial scales. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15360–15365. doi: 10.1073/pnas.1210490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthey A.J.R., Banks P.B. Naivete is not forever: responses of a vulnerable native rodent to its long term alien predators. Oikos. 2016;125:918–926. [Google Scholar]
- Caruso N., Manfredi C., Vidal E.M.L., Casanaveo E.B., Lucherinio M. First density estimation of two sympatric small cats, Leopardus colocolo and Leopardus geoffroyi, in a shrubland area of Central Argentina. Ann. Zool. Fenn. 2012;49:181–191. [Google Scholar]
- Cassano C.R., Barlow J., Pardini R. Large mammals in an agroforestry mosaic in the Brazilian Atlantic forest. Biotropica. 2012;44:818–825. [Google Scholar]
- Chandler R.B., Royle J.A. Spatially explicit models for inference about density in unmarked or partially marked populations. Ann. Appl. Stat. 2013;7:936–954. [Google Scholar]
- Chynoweth M.W., Çoban E., Şekercioğlu C.H. Conservation of a new breeding population of Caucasian lynx (Lynx lynx dinniki) in eastern Turkey. Turk. J. Zool. 2015;39:541–543. [Google Scholar]
- Corlett R.T., Primack R.B., Devictor V., Maas B., Goswami V.R., Bates A.E., Koh L.P., Regan T.J., Loyola R., Pakeman R.J. Impacts of the coronavirus pandemic on biodiversity conservation. Biol. Conserv. 2020;246:108571. doi: 10.1016/j.biocon.2020.108571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove M.V., Kays R., Bontrager H., Bresnen T., Frerichs R.K., McShea W.J., et al. SNAPSHOT USA 2019: the first coordinated national camera trap survey of the United States. Ecology. 2021 doi: 10.1002/ecy.3353. In press. [DOI] [PubMed] [Google Scholar]
- Di Bitetti M.S., De Angelo C.D., Di Blanco Y.E., Paviolo A. Niche partitioning and species coexistence in a Neotropical felid assemblage. Acta Oecol. 2010;36:403–412. [Google Scholar]
- Dillon A., Kelly M.J. Ocelot Leopardus pardalis in Belize: the impact of trap spacing and distance moved on density estimates. Oryx. 2007;41:469–477. [Google Scholar]
- Dillon A., Kelly M.J. Ocelot home range, overlap and density: comparing radio telemetry with camera trapping. J. Zool. 2008;275:391–398. [Google Scholar]
- Ditmer M.A., Stoner D.C., Francis C.D., Barber J.R., Forester J.D., Choate D.M., Ironside K.E., Lonsghore K.M., Hersey K.R., Larsen R.T., McMillan B.R., Olsen D.D., Andreasen A.M., Beckmann J.P., Holton P.B., Messmer T.A., Carter N.H. Artificial nightlight alters the predator-prey dynamics of an apex carnivore. Ecography. 2020;43:1–13. [Google Scholar]
- Dobson A., Nowak K. Does this photo make my range look big? Anim. Conserv. 2010;13:347–349. [Google Scholar]
- Efford M.G., Boulanger J. Fast evaluation of study designs for spatially explicit capture-recapture. Methods Ecol. Evol. 2019;10:1529–1535. [Google Scholar]
- Erb P.L., McShea W.J., Guralnick R.P. Anthropogenic influences on macro-level mammal occupancy in the Appalachian Trail corridor. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espartosa K.D., Pinotti B.T., Pardini R. Performance of camera trapping and track counts for surveying large mammals in rainforest remnants. Biodivers. Conserv. 2011;20:2815–2829. [Google Scholar]
- Farhadinia M.S., Mahdavi A., Hosseini-Zavarei F. Reproductive ecology of the Persian leopard, Panthera pardus saxicolor, in Sarigol National Park, northeastern Iran. Zool. Middle East. 2009;48:13–16. [Google Scholar]
- Fegraus E.H., Lin K., Ahumada J.A., Baru C., Chandra S., Youn C. Data acquisition and management software for camera trap data: a case study from the TEAM network. Ecol. Inform. 2011;6:345–353. [Google Scholar]
- Feinberg J.A., Newman C.E., Watkins-Colwell G.J., Schlesinger M.D., Zarate B., Curry B.R., Shaffer H.B., Burger J. Cryptic diversity in metropolis: confirmation of a new leopard frog species (Anura: Ranidae) from New York City and surrounding Atlantic coast regions. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidino M., Gallo T., Lehrer E.W., Murray M.H., Kay C., Sander H.A., MacDougall B., Salsbury C.M., Ryan T.J., Angstmann J.L., Belaire J.A., Dugelby B., Schell C., Stankowich T., Amaya M., Drake D., Hursh S.H., Ahlers A.A., Williamson J., Hartley L.M., Zellmer A.J., Simon K., Magle S.B. Landscape-scale differences among cities alter common species’ responses to urbanization. Ecol. Appl. 2020:e2253. doi: 10.1002/eap.2253. [DOI] [PubMed] [Google Scholar]
- Ford A.T., Clevenger A.P. Validity of the prey-trap hypothesis for carnivore-ungulate interactions at wildlife-crossing structures. Conserv. Biol. 2010;24:1679–1685. doi: 10.1111/j.1523-1739.2010.01564.x. [DOI] [PubMed] [Google Scholar]
- Ford A.T., Clevenger A.P., Bennett A. Comparison of methods of monitoring wildlife crossing-structures on highways. J. Wildl. Manag. 2009;73:1213–1222. [Google Scholar]
- Foster R.J., Harmsen B.J. A critique of density estimation from camera-trap data. J. Wildl. Manag. 2012;76:224–236. [Google Scholar]
- Foster V.C., Sarmento P., Sollmann R., Tôrres N., Jácomo A.T.A., Negrões N., Fonseca C., Silveira L. Jaguar and puma activity patterns and predator-prey interactions in four Brazilian biomes. Biotropica. 2013;45:373–379. [Google Scholar]
- Frey S., Fisher J.T., Burton A.C., Volpe J.P. Investigating animal activity patterns and temporal partitioning using camera-trap data: challenges and opportunities. Remote Sens. Ecol. Conserv. 2017;3:123–132. [Google Scholar]
- Galaverni M., Palumbo D., Fabbri E., Caniglia R., Greco C., Randi E. Monitoring wolves (Canis lupus) by non-invasive genetics and camera trapping: a small-scale pilot study. Eur. J. Wildl. Res. 2011;58:47–58. [Google Scholar]
- Gallo T., Fidino M., Lehrer E.W., Magle S.B. Mammal diversity and metacommunity dynamics in urban green spaces: implications for urban wildlife conservation. Ecol. Appl. 2017;27:2330–2341. doi: 10.1002/eap.1611. [DOI] [PubMed] [Google Scholar]
- Gallo T., Fidino M., Lehrer E.W., Magle S. Urbanization alters predator-avoidance behaviours. J. Appl. Ecol. 2019;88:793–803. doi: 10.1111/1365-2656.12967. [DOI] [PubMed] [Google Scholar]
- Gardner B., Reppucci J., Lucherini M., Royle J.A. Spatially explicit inference for open populations: estimating demographic parameters from camera-trap studies. Ecology. 2010;91:3376–3383. doi: 10.1890/09-0804.1. [DOI] [PubMed] [Google Scholar]
- Garrard G.E., Bekessy S.A., McCarthy M.A., Wintle B.A. When have we looked hard enough? A novel method for setting minimum survey effort protocols for flora surveys. Austral. Ecol. 2008;33:986–998. [Google Scholar]
- Gaynor K.M., Hojnowski C.E., Carter N.H., Brashares J.S. The influence of human disturbance on wildlife nocturnality. Science. 2018;360:1232–1235. doi: 10.1126/science.aar7121. [DOI] [PubMed] [Google Scholar]
- Gerrodette T. A power analysis for detection trends. Ecology. 1987;68:1364–1372. [Google Scholar]
- Gilbert N.A., Clare J.D.J., Stenglein J.L., Zuckerberg B. Abundance estimation of unmarked animals based on camera-trap data. Conserv. Biol. 2020 doi: 10.1111/cobi.13517. [DOI] [PubMed] [Google Scholar]
- Gompper M.E., Kays R.W., Ray J.C., LaPoint S.D., Bogan D.A., Cryan J.R. A comparison of noninvasive techniques to survey carnivore communities in Northeastern North America. Wildl. Soc. Bull. 2006;34:1142–1151. [Google Scholar]
- Gopalaswamy A.M., Karanth K.U., Kumar N.S., Macdonald D.W. Estimating tropical forest ungulate densities from sign surveys using abundance models of occupancy. Anim. Conserv. 2012;15:669–679. [Google Scholar]
- Gopalaswamy A.M., Royle J.A., Delampady M., Nichols J.D., Karanth K.U., Macdonald D.W. Density estimation in tiger populations: combining information for strong inference. Ecology. 2012;93:1741–1751. doi: 10.1890/11-2110.1. [DOI] [PubMed] [Google Scholar]
- Green A.M., Chynoweth M.W., Şekercioğlu Ç.H. Spatially explicit capture-recapture through camera trapping: a review of benchmark analyses for wildlife density estimation. Front. Ecol. Evol. 2020;8:563477. [Google Scholar]
- Guillera-Arroita G., Ridout M.S., Morgan J.T. Design of occupancy studies with imperfect detection. Methods Ecol. Evol. 2010;1:131–139. [Google Scholar]
- Gutiérrez-González C.E., Gómez-Ramírez M.A., López-González C.A. Estimation of the density of the near threatened jaguar Panthera onca in Sonora, Mexico, using camera trapping and an open population model. Oryx. 2012;46:431–437. [Google Scholar]
- Hackett H.M., Lesmeister D.B., Desanty-Combes J., Montague W.G., Millspaugh J.J., Gompper M.E. Detection rates of eastern spotted skunks (Spilogale putorius) in Missouri and Arkansas using live-capture and non-invasive techniques. Am. Midl. Nat. 2007;158:123–131. [Google Scholar]
- Harihar A., Pandav B., Goyal S.P. Responses of tiger (Panthera tigris) and their prey to removal of anthropogenic influences in Rajaji National Park, India. Eur. J. Wildl. Res. 2009;55:97–105. [Google Scholar]
- Harihar A., Chanchani P., Sharma R.K., Vattakaven J., Gubbi S., Pandav B., Noon B. Conflating “co-occurrence” with “coexistence”. Proc. Natl. Acad. Sci. U. S. Am. 2013;110:E109. doi: 10.1073/pnas.1217001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.L., Barr D.J., Dragoo J. A comparison of population survey techniques for swift foxes (Vulpes velox) in New Mexico. Am. Midl. Nat. 2002;148:320. [Google Scholar]
- Hartop E.A., Brown B.V., Disney R.H.L. Opportunity in our ignorance: urban biodiversity study reveals 20 new species and one new nearctic record for Megaselia (Diptera: Phoridae) in Los Angeles (California, USA) Zootaxa. 2015;3941:451–484. doi: 10.11646/zootaxa.3941.4.1. [DOI] [PubMed] [Google Scholar]
- Hebblewhite M., Haydon D.T. Distinguishing technology from biology: a critical review of the use of GPS telemetry data in ecology. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2010;365:2303–2312. doi: 10.1098/rstb.2010.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R.M., Roe J.D., Wangchuk R., Hunter D.O. Estimating snow leopard population abundance using photography and capture–recapture techniques. Wildl. Soc. Bull. 2006;34:772–781. [Google Scholar]
- Jenks K.E., Chanteap P., Damrongchainarong K., Cutter P., Cutter P., Redford T., Lynam A.J., Howard J., Leimgruber P. Using relative abundance indices from camera-trapping to test wildlife conservation hypotheses – an example from Khao Yai National Park, Thailand. Trop. Conserv. Sci. 2011;4:113–131. [Google Scholar]
- Jenks K.E., Howard J., Leimgruber P. Do ranger stations deter poaching activity in national parks in Thailand? Biotropica. 2012;44:826–833. [Google Scholar]
- Jennelle C.S., Runge M.C., MacKenzie D.I. The use of photographic rates to estimate densities of tigers and other cryptic mammals: a comment on misleading conclusions. Anim. Conserv. 2002;5:119–120. [Google Scholar]
- Jhala Y., Qureshi Q., Gopal R. Can the abundance of tigers be assessed from their signs? J. Appl. Ecol. 2011;48:14–24. [Google Scholar]
- Jimenez J., Nunez-Arjona J.C., Rueda C., Gonzalez L.M., Garcia-Dominguez F., Munoz-Igualada J., Lopez-Bao J.V. Estimating carnivore community structures. Science. 2017;7:41036. doi: 10.1038/srep41036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.P.G. Monitoring species abundance and distribution at the landscape scale. J. Appl. Ecol. 2011;48:9–13. [Google Scholar]
- Jones J.P.G., Asner G.P., And S.H.M.B., Karanth K.U. In: Key Topics in Conservation Ecology. First. Macdonald D.W., Willis K.J., editors. John Wiley and Sons, Ltd.; 2013. The “why”, “what” and “how” of monitoring for conservation; pp. 329–343. [Google Scholar]
- Jordan M.J., Barrett R.H., Purcell K.L. Camera trapping estimates of density and survival of fishers Martes pennanti. Wildl. Biol. 2011;17:266–276. [Google Scholar]
- Kalle R., Ramesh T., Qureshi Q., Sankar K. Density of tiger and leopard in a tropical deciduous forest of Mudumalai Tiger Reserve, southern India, as estimated using photographic capture–recapture sampling. Acta Theriol. 2011;56:335–342. [Google Scholar]
- Karanth K.U. Estimating tiger Panthera tigris populations from camera-trap data using capture—recapture models. Biol. Conserv. 1995;71:333–338. [Google Scholar]
- Karanth K.U., Nichols J.D. Estimation of tiger densities in India using photgraphic captures and recaptures. Ecology. 1998;79:2852–2862. [Google Scholar]
- Kays R., Arbogast B.S., Baker-Whatton M., Beirne C., Boone H.M., Bowler M., Burneo S.F., Cove M.V., Ding P., Espinosa S., Goncalves A.L.S., Hansen C.P., Jansen P.A., Kolowski J.M., Knowles T.W., Lima M.G.M., Millspaugh J., McShea W.J., Pacific K., Parsons A.W., Pease B.S., Rovero F., Santos F., Schuttler S.G., Sheil D., Si X., Snider M., Spironello W.R. An empirical evaluation of camera trap study design: how many, how long and when? Methods Ecol. Evol. 2020;11:700–713. [Google Scholar]
- Kinnaird M.F., O'Brien T.G. Effects of private-land use, livestock management, and human tolerance on diversity, distribution, and abundance of large african mammals. Conserv. Biol. 2012;26:1026–1039. doi: 10.1111/j.1523-1739.2012.01942.x. [DOI] [PubMed] [Google Scholar]
- Kleinschroth F., Kowarik I. COVID-19 crisis demonstrates the urgent need for urban greenspaces. Front. Ecol. Environ. 2020;18:318–319. doi: 10.1002/fee.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouakou C.Y., Boesch C., Kuehl H.S. Identifying hotspots of chimpanzee group activity from transect surveys in Taï National Park, Côte d'Ivoire. J. Trop. Ecol. 2011;27:621–630. [Google Scholar]
- Larrucea E.S., Brussard P.F., Jaeger M.M., Barrett R.H. Cameras, coyotes, and the assumption of equal detectability. J. Wildl. Manag. 2007;71:1682–1689. [Google Scholar]
- Leuchtenberger C., Zucco C.A., Ribas C., Magnusson W., Mourao G. Activity patterns of giant otters recorded by telemetry and camera traps. Ethol. Ecol. Evol. 2014;26:19–28. [Google Scholar]
- Lhota S., Loken B., Spehar S., Fell E., Pospěch A., Kasyanto N. Discovery of Miller's grizzled langur (Presbytis hosei canicrus) in Wehea forest confirms the continued existence and extends known geographical range of an endangered primate. Am. J. Primatol. 2012;74:193–198. doi: 10.1002/ajp.21983. [DOI] [PubMed] [Google Scholar]
- Li S., McShea W.J., Wang D., Lu Z., Gu X. Gauging the impact of management expertise on the distribution of large mammals across protected areas. Divers. Distrib. 2012;18:1166–1176. [Google Scholar]
- Linkie M., Ridout M.S. Assessing tiger-prey interactions in Sumatran rainforests. J. Zool. 2011;284:224–229. [Google Scholar]
- Linkie M., Guillera-Arroita G., Smith J., Rayan D.M. Monitoring tigers with confidence. Integrat. Zool. 2010;5:342–350. doi: 10.1111/j.1749-4877.2010.00215.x. [DOI] [PubMed] [Google Scholar]
- Maas B., Grogan K.E., Chirango Y., Harris N., Lievano-Latorre L.F., McGuire K.L., Moore A.C., Ocampo-Ariza C., Palta M.M., Perfecto I., Primack R.B., Rowell K., Sales L., Santos-Silva R., Aparecida S., Sterling E.J., Vieira R.R.S., Wyborn C., Toomey A. Academic leaders must support inclusive scientific communities during COVID-19. Nat. Ecol. Evol. 2020;4:997–998. doi: 10.1038/s41559-020-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie D.I., Royle J.A. Designing occupancy studies: general advice and allocating survey effort. J. Appl. Ecol. 2005;42:1105–1114. [Google Scholar]
- MacKenzie D.I., Nichols J.D., Lachman G.B., Droege S., Royle J.A., Langtimm C.A. Estimating site occupancy rates when detection probabilities are less than one. Ecology. 2002;83:2248–2255. [Google Scholar]
- MacKenzie D.I., Nichols J.D., Royle J.A., Pollock K.H., Bailey L.L., Hines J.E. Elsevier; London, United Kingdom: 2017. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence. [Google Scholar]
- Magle S.B., Hunt V.M., Vernon M., Crooks K.R. Urban wildlife research: past, present, and future. Biol. Conserv. 2012;155:23–32. [Google Scholar]
- Magle S.B., Fidino M., Lehrer E.W., Gallo T., Mulligan M.P., Rios M.J., Ahlers A.A., Angstmann J., Belaire A., Dugelby B., Gramza A., Hartley L., MacDougall B., Ryan T., Salsbury C., Sander H., Schell C., Simon K., St Onge S., Drake D. Advancing urban wildlife research through multi-city collaboration. Front. Ecol. Environ. 2019;17:232–239. [Google Scholar]
- Main M.B., Richardson L.W. Habitat management response of wildlife to prescribed fire in southwest Florida pine flatwoods. Wildl. Soc. Bull. 2002;30:213–221. [Google Scholar]
- Manzo E., Bartolommei P., Rowcliffe J.M., Cozzolino R. Estimation of population density of European pine marten in central Italy using camera trapping. Acta Theriol. 2012;57:165–172. [Google Scholar]
- Martinuzzi S., Stewart S.I., Helmers D.P., Mockrin M.H., Hammer R.B., Radelhoff V.C. U.S. Department of Agriculture Forest Service; Washington, D.C., USA: 2015. The 2010 Wildland-urban Interface of the Conterminous United States. [Google Scholar]
- Mazzolli M. Mosaics of exotic forest plantations and native forests as habitat of pumas. Environ. Manag. 2010;46:237–253. doi: 10.1007/s00267-010-9528-9. [DOI] [PubMed] [Google Scholar]
- McCallum J. Changing use of camera traps in mammalian field research: habitats, taxa and study types. Mammal Rev. 2013;43:196–206. [Google Scholar]
- McClintock B.T., Conn P., Alonso R., Crooks K.R. Integrated modeling of bilateral photo-identification data in mark-recapture analyses. Ecology. 2013;94:1464–1471. doi: 10.1890/12-1613.1. (130315134921006) [DOI] [PubMed] [Google Scholar]
- McNamara J., Robinson E.J.Z., Abernathy K., Iponga D.M., Sackey H.N.K., Wright J.H., Milner-Gulland E.J. COVID-19, systemic crisis, and possible implications for the wild meat trade in sub-Saharran Africa. Environ. Resour. Econ. 2020;76:1045–1066. doi: 10.1007/s10640-020-00474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek P.D., Ballard G., Claridge A., Kays R., Moseby K., O'Brien T., O'Connell A., Sanderson J., Swann D.E., Tobler M., Townsend S. Recommended guiding principles for reporting on camera trapping research. Biodivers. Conserv. 2014;23:2321–2343. [Google Scholar]
- Meijaard E., Chener A.C.K.I.T., Smeenk C. “New Bornean carnivore” is most likely a little known flying squirrel. Mammal Rev. 2006;36:318–324. [Google Scholar]
- Mohamed A., Sollmann R., Bernard H., Ambu L.N., Lagan P., Mannan S., Hofer H., Wilting A. Density and habitat use of the leopard cat (Prionailurus bengalensis) in three commercial forest reserves in Sabah, Malaysian Borneo. J. Mammal. 2013;94:82–89. [Google Scholar]
- Muhly T.B., Semeniuk C., Massolo A., Hickman L., Musiani M. Human activity helps prey win the predator-prey space race. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi M., Coban E., Kusak J., Kemahli Aytekin M.C., Chynoweth M., Agirkaya I.K., Guven N., Coban A., Sekercioglu C.H. The first record of raccoon dog (Nyctereutes procyonoides) in Turkey. Turk. J. Zool. 2020;44:209–213. [Google Scholar]
- Naidoo R., Burton A.C. Relative effects of recreational activities on a temperate terrestrial wildlife assemblage. Conserv. Sci. Pract. 2020;2 [Google Scholar]
- Negrões N., Sollmann R., Fonseca C., Jácomo A.T.A., Revilla E., Silveira L. One or two cameras per station? Monitoring jaguars and other mammals in the Amazon. Ecol. Res. 2012;27:639–648. [Google Scholar]
- Nichols J.D., Karanth K.U., O'Connell A.F. In: Camera Traps in Animal Ecology. O'Connell A.F., Nichols J.D., Karanth K.U., editors. Springer; Japan, Tokyo: 2011. Science, conservation, and camera traps; pp. 45–56. [Google Scholar]
- Núñez-Pérez R. Estimating jaguar population density using camera-traps: a comparison with radio-telemetry estimates. J. Zool. 2011;285 [Google Scholar]
- Obbard M.E., Howe E.J., Kyle C.J. Empirical comparison of density estimators for large carnivores. J. Appl. Ecol. 2010;47:76–84. [Google Scholar]
- O'Brien T.G. In: Camera Traps in Animal Ecology. O'Connell A.F., Nichols J.D., Karanth K.U., editors. Springer; Tokyo, Japan: 2011. Abundance, density, and relative abundance; pp. 71–96. [Google Scholar]
- O'Brien T.G., Kinnaird M.F. Density estimation of sympatric carnivores using spatially explicit capture–recapture methods and standard trapping grid. Ecol. Appl. 2011;21:2908–2916. [Google Scholar]
- O'Brien T.G., Kinnaird M.F., Wibisono H.T. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. 2003;6:131–139. [Google Scholar]
- O'Connell A.F., Bailey L.L. In: Camera Traps in Animal Ecology. O'Connell A.F., Nichols J.D., Karanth K.U., editors. Springer; Tokyo, Japan: 2011. Inference for occupancy and occupancy dynamics; pp. 191–205. [Google Scholar]
- O'Connell A.F., Talancy N.W., Bailey L.L., Sauer J.R., Cook R., Gilbert A.T. Estimating site occupancy and detection probability parameters for meso- and large mammals in a coastal ecosystem. J. Wildl. Manag. 2006;70:1625–1633. [Google Scholar]
- O'Connell A.F., Nichols J.D., Karanth U.K. Springer Japan; Tokyo: 2011. Camera Traps in Animal Ecology. [Google Scholar]
- Oliveira-Santos L.G.R., Zucco C.A., Antunes P.C., Crawshaw P.G. Is it possible to individually identify mammals with no natural markings using camera-traps? A controlled case-study with lowland tapirs. Mammal. Biol. Z. Säugetierkunde. 2010;75:375–378. [Google Scholar]
- Ordeñana M.A., Crooks K.R., Boydston E.E., Fisher R.N., Lyren L.M., Siudyla S., Haas C.D., Harris S., Hathaway S.A., Turschak G.M., Miles A.K., Van Vuren D.H. Effects of urbanization on carnivore species distribution and richness. J. Mammal. 2010;91:1322–1331. [Google Scholar]
- Palomares F., Godoy J.A., López-Bao J.V., Rodríguez A., Roques S., Casas-Marce M., Revilla E., Delibes M. Possible extinction vortex for a population of Iberian lynx on the verge of extirpation. Conserv. Biol. 2012;26:689–697. doi: 10.1111/j.1523-1739.2012.01870.x. [DOI] [PubMed] [Google Scholar]
- Parsons A.W., Bland C., Forrester T., Baker-Whatton M.C., Schuttler S.G., McShea W.J., Costello R., Kays R. The ecological impact of humans and dogs on wildlife in protected areas in eastern North America. Biol. Conserv. 2016;203:75–88. [Google Scholar]
- Parsons A.W., Forrester T., Baker-Whatton M.C., McShea W.J., Rota C.T., Schuttler S.G., Millspaugh J.J., Kays R. Mammal communities are larger and more diverse in moderately developed areas. eLife. 2018;7 doi: 10.7554/eLife.38012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A.W., Rota C.T., Forrester T., Baker-Whatton M.C., McShea W.J., Schuttler S.G., Millspaugh J.J., Kays R. Urbanization focuses carnivore activity in reamining natural habitats, increasing species interaction. J. Appl. Ecol. 2019 doi: 10.1111/1365-2664.13385. [DOI] [Google Scholar]
- Paull D.J., Claridge A.W., Cunningham R.B. Effective detection methods for medium-sized ground dwelling mammals: a comparison between infrared digital cameras and hair tunnels. Wildl. Res. 2012;39:546–553. [Google Scholar]
- Pennisi E. Pandemic robs field scientists of ‘once-in-a-lifetime’ moments. Science. 2020;15(April) [Google Scholar]
- Public Broadcasting Service (PBS) Observing a brown bear's daily routine with Cagan Sekercioglu. 2018. https://www.pbs.org/wnet/nature/animals-cameras-observing-brown-bears-daily-routine-cagan-sekercioglu/16063/ URL: (Arlington, Virginia)
- Ramesh T., Kalle R. Activity pattern of sloth bear Melursus ursinus (Mammalia: Ursidae) in Mudumalai Tiger Reserve, Western Ghats, India. J. Theatened Taxa. 2013;5:3989–3992. [Google Scholar]
- Ramesh T., Kalle R., Sankar K., Qureshi Q. Spatio-temporal partitioning among large carnivores in relation to major prey species in Western Ghats. J. Zool. 2012;287:269–275. [Google Scholar]
- Razani N., Radhakrishna R., Chan C. Public lands are essential to public health during a pandemic. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1271. [DOI] [PubMed] [Google Scholar]
- Rice W.L., Mateer T.J., Reigner N., Newman P., Lawhon B., Taff B.D. Changes in recreational behaviors of outdoor enthusiasts during the COVID-19 pandemic: analysis across urban and rural communities. J. Urban Ecol. 2020 doi: 10.31235/osf.io/prnz9. [DOI] [Google Scholar]
- Rich L.N., Davis C.L., Farris Z.J., Miller D.A.W., Tucker J.M., Hamel S., Farhadinia M.S., Steenweg R., Di Bitetti M.S., Thapa K., Kane M.D., Sunarto S., Robinson N.P., Paviolo A., Cruz P., Martins Q., Gholikhani N., Taktehrani A., Whittington J., Widodo F.A., Yoccoz N.G., Wultsch C., Harmsen B.J., Kelly M.J. Assessing global patterns in mammalian carnivore occupancy and richness by integrating local camera trap surveys. Glob. Ecol. Biogeogr. 2017;26:918–929. [Google Scholar]
- Rich L.N., Miller D.A.W., Robinson H.S., McNutt J.W., Kelly M.J. Using camera trapping and hierarchical occupancy modelling to evaluate the spatial ecology of an African mammal community. J. Appl. Ecol. 2016;53:1225–1235. [Google Scholar]
- Ripple W.J., Estes J.A., Beschta R.L., Wilmers C.C., Ritchie E.G., Hebblewhite M., et al. Status and ecological effects of the world's largest carnivores. Science. 2014;343:1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- Rondinini C., Rodrigues A.S.L., Boitani L. The key elements of a comprehensive global mammal conservation strategy. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2011;366:2591–2597. doi: 10.1098/rstb.2011.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Rosas O.C., Bender L.C. Population status of jaguars (Panthera onca) and pumas (Puma concolor) in Northeastern Sonora, Mexico. Acta Zool. Mexic. 2012;28:86–101. [Google Scholar]
- Rota C.T., Ferreira M.A.R., Kays R.W., Forrester T.D., Kalies E.L., McShea W.J., Parsons A.W., Millspaugh J.J. A multispecies occupancy model for two or more interacting species. Methods Ecol. Evol. 2016;7:1164–1173. [Google Scholar]
- Rovero F., Rathbun G.B., Perkin A., Jones T., Ribble D.O., Leonard C., Mwakisoma R.R., Doggart N. A new species of giant sengi or elephant-shrew (genus Rhynchocyon) highlights the exceptional biodiversity of the Udzungwa Mountains of Tanzania. J. Zool. 2008;274:126–133. [Google Scholar]
- Rovero R., Zimmerman F., Berzi D., Meek P. “Which camera trap type and how many do I need?” A review of camera features and study designs for a range of wildlife research applications. Hystrix Ital. J. Mammal. 2013;24:148–156. [Google Scholar]
- Rowcliffe J.M. Key frontiers in camera trapping research. Remote Sens. Ecol. Conserv. 2017;3:107–108. [Google Scholar]
- Rowcliffe J.M., Field J., Turvey S.T., Carbone C. Estimating animal density using camera traps without the need for individual recognition. J. Appl. Ecol. 2008;45:1228–1236. [Google Scholar]
- Royle J.A., Nichols J.D., Karanth K.U., Gopalaswamy A.M. A hierarchical model for estimating density in camera-trap studies. J. Appl. Ecol. 2009;46:118–127. [Google Scholar]
- Royle J.A., Chandler R.B., Sollmann R., Gardner B. Academic Press; Waltham: 2014. Spatial Capture-Recapture. [Google Scholar]
- Royle J.A., Fuller A.K., Sutherland C. Unifying population and landscape ecology with spatial capture-recapture. Ecography. 2018;41:444–456. [Google Scholar]
- Rutz C., Loretto M., Bates A.E., Davidson S.C., Duarte C.M., Jetz W., Johnson M., Kato A., Kays R., Mueller T., Primack R.B., Ropert-Coudert Y., Tucker M.A., Wikelski M., Cagnacci F. COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nat. Ecol. Evol. 2020;4:1156–1159. doi: 10.1038/s41559-020-1237-z. [DOI] [PubMed] [Google Scholar]
- Sahagun L. Los Angeles Times; 2020. Coyotes, falcons, deer and other wildlife are reclaiming L.A. territory as humans stay at home. https://www.latimes.com/environment/story/2020-04-21/wildlife-thrives-amid-conronavirus-lockdown
- Samuelsson K., Barthel S., Colding J., Macassa G., Giusti M. 2020. Urban Nature as a Source of Resilience During Social Distancing Amidst the Coronavirus Pandemic. (OSF Preprints. doi.org/10.31219.osf.io/3wx5a) [Google Scholar]
- Saraswat R., Saraswat D.A. Research opportunities in pandemic lockdown. Science. 2020;368:594–595. doi: 10.1126/science.abc3372. [DOI] [PubMed] [Google Scholar]
- Schuette P., Wagner A.P., Wagner M.E., Creel S. Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biol. Conserv. 2013;158:301–312. [Google Scholar]
- Şekercioğlu Ç.H., Anderson S., Akçay E., Bilgin R., Can Ö.E., Semiz C., Tavşanoğlu Ç., Yokeş M.B., Soyumert A., İpekdal K., Sağlam İ.K., Yücel M., Nüzhet Dalfes H. Turkey's globally important biodiversity in crisis. Biol. Conserv. 2011;144:2752–2769. [Google Scholar]
- Sekercioglu C. 2013. Happy 125th National Geographic: Brown Bears Film Their Lives with Turkey’s First CritterCams.http://newswatch.nationalgeographic.com/2013/01/13/happy-125th-national-geographic-brown-bears-film-their-lives-with-turkeys-first-crittercams/ [Google Scholar]
- Sharma R.K., Jhala Y., Qureshi Q., Vattakaven J., Gopal R., Nayak K. Evaluating capture-recapture population and density estimation of tigers in a population with known parameters. Anim. Conserv. 2010;13:94–103. [Google Scholar]
- Silva-Rodríguez E.A., Sieving K.E. Domestic dogs shape the landscape-scale distribution of a threatened forest ungulate. Biol. Conserv. 2012;150:103–110. [Google Scholar]
- Singh P., Gopalaswamy A.M., Karanth K.U. Factors influencing densities of striped hyenas (Hyaena hyaena) in arid regions of India. J. Mammal. 2010;91:1152–1159. [Google Scholar]
- Slater S.J., Christiana R.W., Gustat J. Recommendations for keeping parks and green space accessible for mental and physical health during COVID-19 and other pandemics. Prevent. Chronic Dis. 2020;17:E59. doi: 10.5888/pcd17.200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soisalo M.K., Cavalcanti S.M.C. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture–recapture sampling in combination with GPS radio-telemetry. Biol. Conserv. 2006;129:487–496. [Google Scholar]
- Sollmann R., Mohamed A., Samejima H., Wilting A. Risky business or simple solution - relative abundance indices from camera-trapping. Biol. Conserv. 2013;159:405–412. [Google Scholar]
- Sollmann R., Gardner B., Chandler R.B., Shindle D.B., Onorato D.P., Royle J.A., O'Connell A.F. Using multiple data sources provides density estimates for endangered Florida panther. J. Appl. Ecol. 2013;50:961–968. [Google Scholar]
- Stafford R., Lloyd J.R. Evaluating a Bayesian approach to improve accuracy of individual photographic identification methods using ecological distribution data. Comput. Ecol. Softw. 2011;1:49–54. [Google Scholar]
- Steenweg R., Whittington J., Hebblewhite M., Forshner A., Johnston B., Petersen D., Shepherd B., Lukacs P.M. Camera-based occupancy monitoring at large scales: power to detect trends in grizzly bears across the Canadian Rockies. Biol. Conserv. 2016;201:192–200. [Google Scholar]
- Steenweg R., Hebblewhite M., Kays R., Ahumada J., Fisher J.T., Burton C., Townsend S.E., Carbone C., Rowcliffe J.M., Whittington J., Brodie J., Royle J.A., Switalski A., Clevenger A.P., Heim N., Rich L.N. Scaling up camera traps: monitoring the planet's biodiversity with networks of remote sensors. Front. Ecol. Environ. 2017;15:26–34. [Google Scholar]
- Tilson R., Defu H., Muntifering J., Nyhus P.J. Dramatic decline of wild South China tigers Panthera tigris amoyensis: field survey of priority tiger reserves. Oryx. 2004;38:40–47. [Google Scholar]
- Trolle M., Noss A.J., Cordeiro J.L.P., Oliveira L.F.B. Brazilian tapir density in the pantanal: a comparison of systematic camera-trapping and line-transect surveys. Biotropica. 2008;40:211–217. [Google Scholar]
- Vilardell A., Capalleras X., Budó J., Pons P. Predator identification and effects of habitat management and fencing on depredation rates of simulated nests of an endangered population of Hermann's tortoises. Eur. J. Wildl. Res. 2012;58:707–713. [Google Scholar]
- Vojta C.D. Old dog new tricks-innovations with presence-absence information. J. Wildl. Manag. 2005;69:845–848. [Google Scholar]
- Waldon J., Miller B.W., Miller C.M. A model biodiversity monitoring protocol for REDD projects. Trop. Conserv. Sci. 2011;4:254–260. [Google Scholar]
- Wang Y., Allen M.L., Wilmers C.C. Mesopredator spatial and temporal responses to larege predators and human development in the Santa Cruz Mountains of California. Biol. Conserv. 2015;190:23–33. [Google Scholar]
- Wearn O.R., Glover-Kapfer P. Snap happy: camera traps are an effect sampling tool when compared with alternative methods. R. Soc. Open Sci. 2019;6:181748. doi: 10.1098/rsos.181748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckel M., Rockwell R.F. Can controlled bow hunts reduce overabundant white-tailed deer populations in suburban ecosystems? Ecol. Model. 2013;250:143–154. [Google Scholar]
- Weckel M., Giuliano W., Silver S. Jaguar (Panthera onca) feeding ecology: distribution of predator and prey through time and space. J. Zool. 2006;270 [Google Scholar]
- Whittington J., Hebblewhite M., Chandler R.B. Generalized spatial mark-resight models with an application to grizzly bears. J. Appl. Ecol. 2018;55:157–168. [Google Scholar]
- Williams B.K., Nichols J.D., Conroy M.J. Academic Press; San Diego, California, USA: 2002. Analysis and Management of Animal Populations. [Google Scholar]
- Yamada F., Kawauchi N., Nakata K., Abe S., Kotaka N., Takashima A., Murata C., Kuroiwa A. Rediscovery after thirty years since the last capture of the critically endangered Okinawa spiny rat Tokudaia muenninki in the northern part of Okinawa Island. Mammal Stud. 2010;35:243–255. [Google Scholar]
- Zellmer A.J., Wood E.M., Surasinghe T., Putman B.J., Pauly G.B., Magle S.B., Lewis J.S., Kay C.A.M., Fidino M. What can we learn from wildlife sightings during the COVID-19 global shutdown? Ecosphere. 2020;11 doi: 10.1002/ecs2.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full dataset of articles included in the systematic review of camera trap literature within the ISI Web of Knowledge Complete Collection.