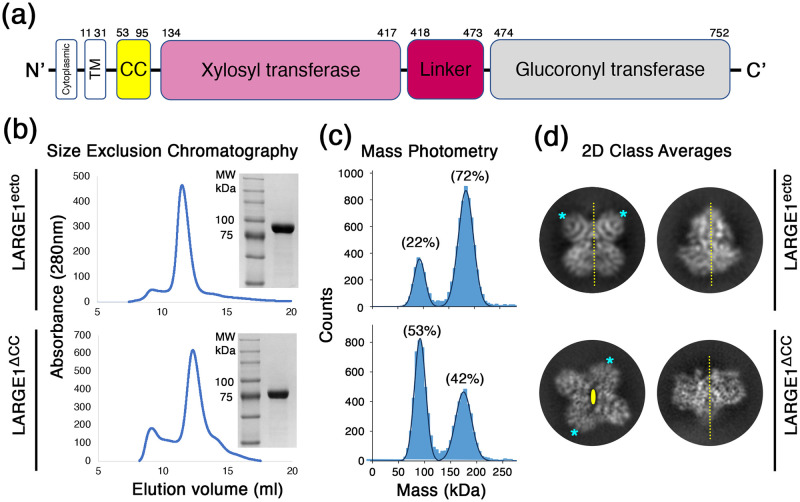
Fig 1. LARGE1 dimerizes in solution.
(a) A schematic diagram for the domain organization in the LARGE1 protein. (b) Purification of the recombinant LARGE1 proteins. Chromatograms from SEC for constructs with (LARGE1ecto, upper image) or without (LARGE1ΔCC, lower image) the CC domain. Insets show Coomassie-stained SDS-PAGE for the purified proteins. (c) Mass-photometry analysis of the two LARGE1 proteins at a concentration of 30 nM. The fraction of counts is noted for the two main peaks in the data sets that corresponds to the monomeric (~80 kDa) and dimeric (~160 kDa) forms of LARGE1. The rest of the counts (6% and 5%, for with and without CC, respectively) do not belong to these two main peaks. These are representative measurements that were repeated multiple times using different protein batches. (d) 2D class averages of LARGE1 particles from the two forms. For each LARGE1 form, two separate classes are shown, representing two distinct and perpendicular views. The 2-fold symmetry axes are shown as dashed yellow lines (for in-plane axes) or as an oval shape (for out-of-plane axis). Same-type domains are marked with asterisks.