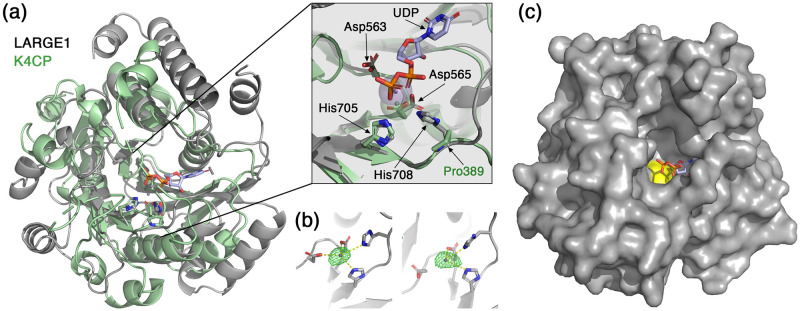
Fig 5. The GlcA-T domain of LARGE1.
(a) Superimposition of the GlcA-T domain from LARGE1 (grey) with K4CP (PDB: 2z86, green). Inset shows a closeup view of the active site. Mn2+ atoms of LARGE1 (purple) and of K4CP (green) are shown as semi-transparent spheres. A UDP from the K4CP structure is shown in purple. The conserved DXD motif and histidine residues that participate in the coordination of the Mn2+ are noted. (b) The GlcA-T active sites of the two LARGE1 monomers (left and right) in the crystallographic asymmetric unit are shown with an Fo-Fc difference map (green mesh, σ = 5) calculated after omitting the Mn2+ atoms. (c) Surface representation of the LARGE1 GlcA-T domain. The aspartic acids of the DXD motif are colored yellow. The UDP derived from the structure of K4CP is shown as sticks.