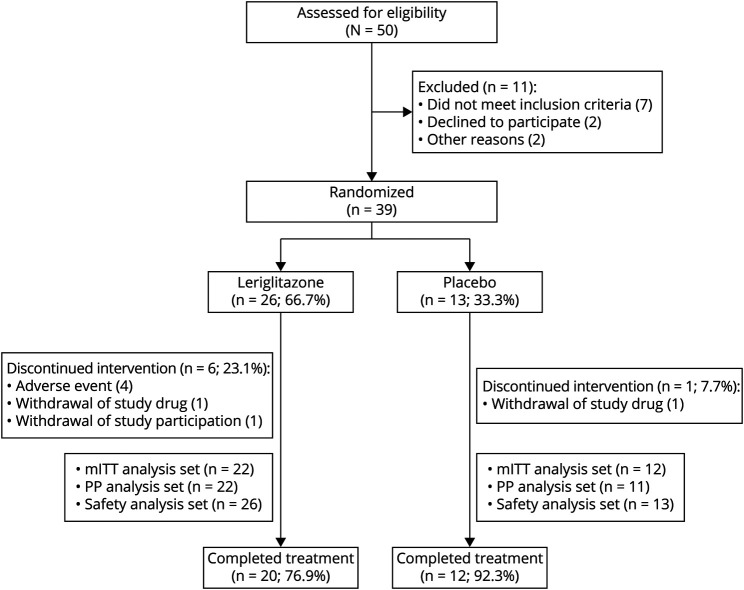
Figure 1. CONSORT Diagram.
The safety analysis set included all randomized patients who received at least 1 dose (partial or complete) of the study drug. The mITT analysis set included all patients who took at least 1 dose (partial or complete) of the study drug and had at least 1 postbaseline spinal cord area segment C2-C3 measurement and SARA assessment at the same visit. The PP analysis set included all patients in the mITT analysis set who did not have a major protocol deviation. CONSORT = Consolidated Standards of Reporting Trials; mITT = modified intent-to-treat; PP = per protocol; SARA = Scale for the Assessment and Rating of Ataxia.