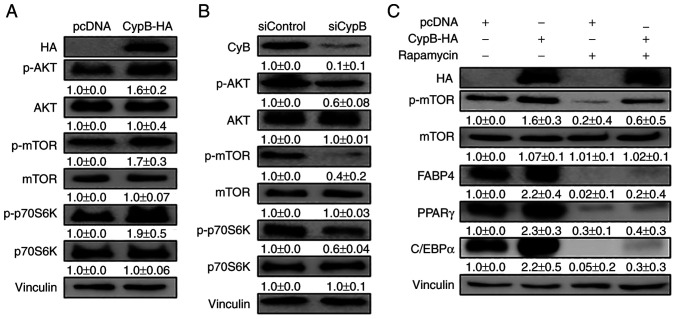
Figure 4.
CypB regulates the AKT/mTOR signaling pathway in 3T3-L1 adipocytes. 3T3-L1 cells at 70-80% confluence were transfected with plasmids or siRNA at 37°C for 48 h. Then the fully confluent cells were differentiated with MDI treatment for 24 h. CypB overexpression and CypB knockdown efficiency were monitored by western blotting. The protein band intensities were quantified by ImageJ and were normalized to the expression of vinculin. (A) Following transfection with CypB-HA or pcDNA, 3T3-L1 preadipocytes were differentiated into adipocytes for 24 h. The expression of AKT/mTOR signaling pathway molecules (p-AKT, p-mTOR and p-p70S6K) was monitored by Western blotting. (B) After gene knockdown by siCypB or siControl, 3T3-L1 preadipocytes were differentiated into adipocytes for 24 h. AKT/mTOR signaling pathway molecules (p-AKT, p-mTOR and p-p70S6K) were monitored by western blotting. (C) Following transfection with CypB-HA or pcDNA, 3T3-L1 preadipocytes were differentiated into adipocytes in the presence or absence of rapamycin and mTOR inhibitor for 7 days and subjected to western blotting with antibodies against late adipogenic factors such as C/EBPα, PPARγ and FABP4. Vinculin was used as a loading control. The control was pcDNA (-rapamycin). The values were shown as the mean ± standard deviation of three independent experiments. CypB, cyclophilin B; AKT, protein kinase B; mTOR, mammalian target of rapamycin; p-phosphorylated; si, short interfering; C/EBP, CCAAT-enhancer binding protein; PPARγ, peroxisome proliferator-activated receptor γ; FABP4, fatty acid binding protein 4; ND, non-differentiation; CON, untreated differentiation control.