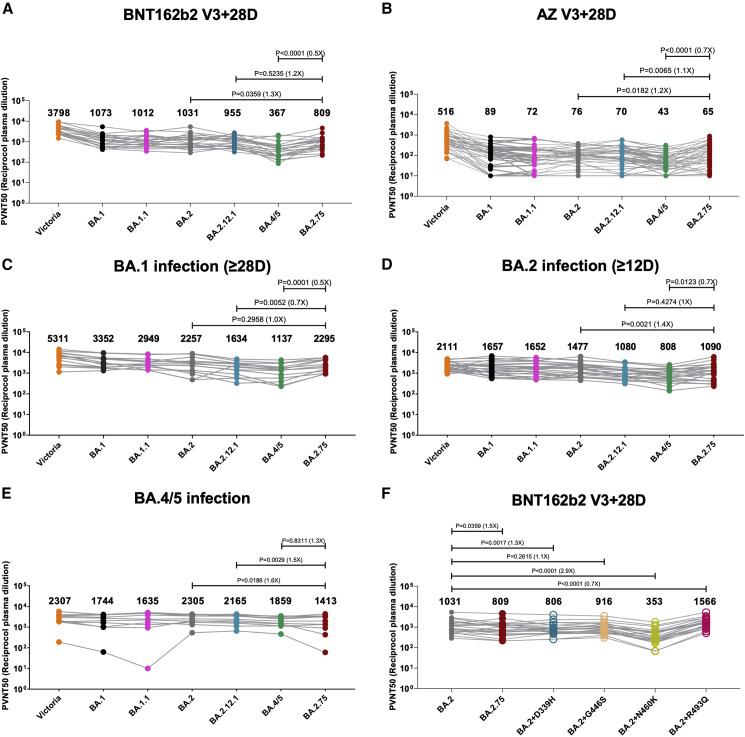
Figure 2.
Pseudoviral neutralization assays of BA.2.75 by vaccine and BA.1, BA.2, and BA.4/5 immune serum
(A and B) IC50 values for the indicated viruses using serum obtained from vaccinees 28 days following their third dose of vaccine (A) Pfizer BNT162b2 (n = 22) or (B) AstraZeneca AZD AZD1222 (n = 41).
(C–E) Serum from volunteers suffering vaccine breakthrough BA.1 (n = 16), BA.2 (n = 23), or BA.4/5 (n = 11) infections.
(F) IC50 values for single RBD point mutations inserted into the BA.2 pseudovirus using Pfizer BNT162b2 serum (n = 22).
Geometric mean titers are shown above each column. The Wilcoxon matched-pairs signed rank test was used for the analysis and two-tailed p values were calculated.
See also Table S3.