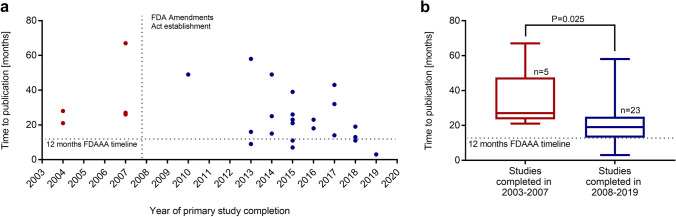
Fig. 3.
a Time from completion to availability of results by year of primary study completion. Each dot represents one completed study. The date of the establishment of the FDA Amendments Act is indicated by the vertical dotted line. The maximal recommended timeframe of 12 months between primary study completion and publication of results as mandated by the FDA is marked by the horizontal dotted line. b Median time to publication of completed studies until 2007 and since 2008 after the establishment of the FDA Amendments Act. The maximal recommended timeframe of 12 months between primary study completion and publication of results as mandated by the FDA is marked by the horizontal dotted line. FDA, Federal Drug Administration