ABSTRACT
There have been previous studies, especially in Western countries and even in some areas in Asia, about extra-intestinal manifestations (EIMs) and its link with the outcome of inflammatory bowel disease (IBD), which includes Crohn’s disease (CD), and ulcerative colitis (UC). This link is crucial when discussing a patient’s prognosis and important when dealing with UC management. The aim of this study was to clarify the most common comorbidities associated with UC, emphasizing immunologic comorbidities in Japan. This study was a retrospective analysis performed at Nagoya University Hospital. The data collection started in March, 2019, and continued for two years. We retrieved the medical records of 105 patients with UC diagnosis, from which the data of 176 EIMs were extracted and analyzed. Results showed that EIMs with UC in the active phase accounted for 43.7% of total EIMs. Twenty-six patients with immune-mediated inflammatory disease frequently had an active phase (odds ratio [OR] 3.84, 99% CI, 1.44–10.27). Comorbidities showing an active manifestation of symptoms and UC in the active phase were significantly correlated in patients with immunological comorbidities, such as peripheral arthritis (r = 0.97, p < 0.01) and rheumatoid arthritis (RA) (r = 0.99, p < 0.01), as well as in patients with primary sclerosis cholangitis (PSC) (r = 0.98, p < 0.01). In conclusion, this analysis suggests the importance of having full comprehension of how immunological comorbidities affect the natural development of UC, which is of vital importance to prevent further UC complications and properly adjust the management of the disease.
Key Words: ulcerative colitis, Crohn’s disease, inflammatory bowel disease, extra-intestinal manifestations, comorbidity
INTRODUCTION
Ulcerative colitis (UC), along with Crohn’s disease (CD), conform to inflammatory bowel disease (IBD), and is a chronic idiopathic inflammatory bowel disease that usually manifests in the colon, causing continuous inflammation, with a span from the rectum to the more proximal colon, and is characterized by a relapsing and remitting course.1 UC can be categorized according to the disease extent (proctitis, left-sided colitis, and extensive colitis) and according to disease severity, expressed in the clinical, laboratory, imaging, and endoscopic parameters.2 Although UC etiopathogenesis is not completely known, immune-related mechanisms are activated in genetically predisposed individuals, triggering a disproportionate immune response against intraluminal antigens.3 Immune reaction in UC is characterized by T-helper 2 (Th2) (humoral), which leads to elevated interleukin-5 (IL-5), which is related to high levels of IgG1.4 In order to provide appropriate treatment, knowing the disease severity (usually classified as remission, mild, moderate, or severe) is essential.5 Thus, for mild to moderate UC, aminosalicylates (5-ASA) are the main choice to be used. For UC flares, topical and systemic steroids are selected, and for moderate to severe disease, immunosuppressants and biological drugs are the main choice.6 In addition, tools such as Mayo score,7 Lichtiger score,8 and Simple Clinical Colitis Activity Index9 are widely used as UC severity indices.2 On the other hand, extra-intestinal manifestations (EIMs), which may occur when primed lymphocytes are in circulation, tend to be a common problem among IBD patients.10 EIMs may occur in various organs, including the joints, skin, liver, eyes, and biliary tracts.11 These EIMs may have different effects on IBD cases, with some responding to treatment from the onset stage, and others to a specific treatment.12 There are some comorbidities or EIMs, including chronic immune-mediated diseases such as erythema nodosum, ankylosing spondylitis (AS), and primary sclerosing cholangitis (PSC), which appear to be most common in IBD.13 Moreover, peripheral arthritis, aphthous stomatitis, and uveitis are also characterized as most frequent EIMs.14 Previous studies have been performed across the world regarding prevalence of EIMs in IBD patients. For instance, in Canada,13 6.2% of IBD patients suffered from at least one EIM, and in Switzerland,14 43% of CD patients and 31% of UC patients had at least one EIM. In Asia, proportions are not constant, and approximately 6% to 14% of IBD patients in East Asia suffer from at least one EIM.15 Although there is limited information regarding EIM in UC patients among the pediatric population in Japan,16 more is required. Thus, the main objective of this study was to clarify the most common comorbidities associated with UC, emphasizing the immunologic comorbidities in Japan.
PATIENTS AND METHODS
This study was conducted in accordance with the Research Ethics Committee of the Graduate School of Medicine (approved ID 2015-0466) at Nagoya University. The procedures followed were in accordance with the Declaration of Helsinki. Due to the retrospective nature of this study, patient consent was waived. Patient information was retrieved using electronic medical records from Nagoya University Hospital, which allowed the identification of individual patients during and/or after data collection. Patient data were anonymized and coded prior to analysis, and data collection began in March, 2019 and finished in January, 2021.
Medical records, including patients’ clinical, pathological, endoscopic, and radiological information, were retrieved retrospectively in all cases. Based on previous studies,17 information regarding date of birth, sex, date of diagnosis (UC and EIM), and treatment for UC and EIM were collected. Information corresponding to patients with a UC diagnosis who had an EIM (comorbidity) in the medical chart was recorded: Mayo Clinic Score was used to categorize UC activity, naming the UC cases fitting into mild, moderate, and severe cases of UC in the active phase, and the remaining cases of UC in remission.18 Furthermore, UC case data were obtained from patients who met the following inclusion criteria: above 18 years of age; diagnosis of UC for more than 3 months before study initiation; Mayo score of 3 to 12 points; and moderate to severe active disease on colonoscopy.19
On the other side of the coin, UC-related comorbidities are defined as complications that are closely associated with active inflammation, which often respond to medical or surgical treatment of the underlying UC. UC-related comorbidities mainly include the joints and skin EIMs.20 According to previous studies, EIMs include diseases such as choledocholithiasis, nephrolithiasis, osteoporosis, uveitis, iritis, erythema nodosum, PSC, rheumatoid arthritis (RA), peripheral arthritis, AS, atopic dermatitis, and pyoderma gangrenosum (PG).10 In addition, immune-related inflammatory diseases (IMID), a group of diseases whose main feature is the presence of systemic inflammation and immune dysregulation,21 described in previous literature are: uveitis, iritis, psoriasis, multiple sclerosis, PSC, erythema nodosum, PG, peripheral arthritis, atopic dermatitis, RA, and AS.13,22,23 Thus, UC immunological comorbidities are considered to be: AS, RA, uveitis, iritis, PSC, erythema nodosum, PG, peripheral arthritis, and atopic dermatitis (Fig. 1). From this group, for the purposes of this study, the following UC immunological comorbidities were analyzed: PSC, peripheral arthritis, RA, AS, PG, and atopic dermatitis (Table 1).
Fig. 1.
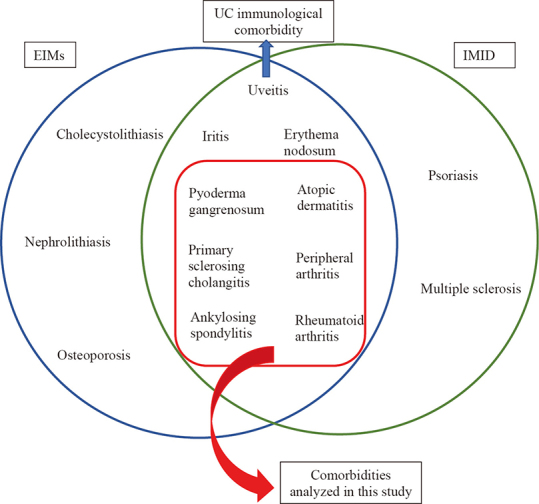
Comorbidities related to UC
This diagram showed the diseases considered as EIMs, IMID and UC immunological comorbidity.
EIMs: extra-intestinal manifestations
IMID: immune-mediated inflammatory disease
UC: ulcerative colitis
Table 1.
International Statistical Classification of Diseases and Related Health Problems (ICD) codes of diseases analyzed in this study
| Disease | ICD |
| Ulcerative colitis, unspecified | K51.9 |
| Polyarthritis, unspecified | K51.9 |
| Rheumatoid arthritis, unspecified | M06.9 |
| Pyoderma gangrenosum | L88 |
| Atopic dermatitis | L20 |
| Primary sclerosing cholangitis | K83.0 |
| Ankylosing spondylitis | M45 |
Comorbidity manifestation was defined as clinical signs and symptoms expressed in an active manner over time.24 We evaluated the UC immunological comorbidity activity using clinical scores of each of the diseases analyzed in this study: for PG, the PARACELSUS score25 was used, in which patients with 10 points or more were considered for this study; for Atopic Dermatitis, the SCORAD score26 was used in which patients fit a moderate and severe score; for PSC patients, clinical symptoms (mainly fatigue, pruritus, or jaundice) and radiographic features (mainly irregular duct contour, multiple intra- and/or extrahepatic bile duct strictures, and saccular ductal dilations) were taken into account.27 In addition, PSC patients who had 14 points or more on the MELD score were included in the study. Finally, for patients who had peripheral arthritis, clinical patterns were considered, that is, joint swelling and joint pain, as well as increased levels of C-reactive protein and/or ESR.28 Furthermore, in RA cases, the DAS28 score was used to assess comorbidity activity, with 2.6 or more considered as active RA. Likewise, increased C-reactive protein levels were taken into account in cases when the erythrocyte sedimentation rate number was not found in the medical chart29,30; and in cases of AS, the ASDAS31 score was used, in which values that were equal or more than 1.3 were considered to participate in this study.
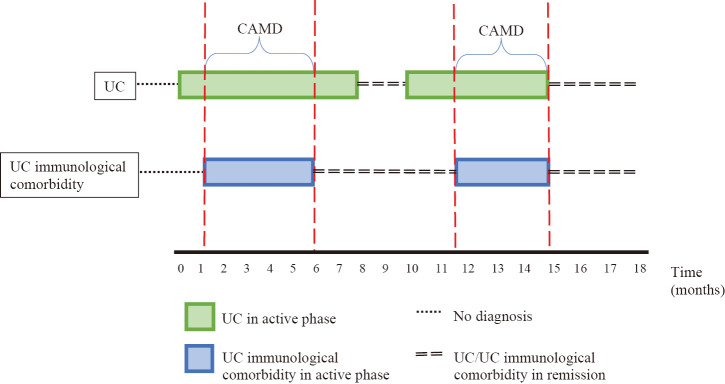
UC and comorbidity manifestations in patients were followed over time using patient information documented previously in medical records. They were tracked as follows: once UC active was described in the medical chart, signs of UC immunological comorbidity activity were sought using the activity scores of each comorbidity. Whenever both the UC and UC immunological comorbidity were found to be in active phase, that patient was tracked during his/her evolution entry by entry in the medical chart, until either UC or UC immunological comorbidity signs of activity disappeared. Even if the active phases on either the UC and UC immunological comorbidity had an intermittent pattern, the active periods were only considered for analysis when both the UC and UC immunological comorbidity were in active phase. The period when both UC activity and UC immunological comorbidity activity overlapped was defined as Common Activity Manifestation Duration (CAMD) (Fig. 2). CAMD of each disease (including UC) was defined as the period in which signs of disease activity took place and was recorded in months. When the CAMD followed and intermittent pattern, those CAMD periods were added together to analyze them. The relationship between UC immunological comorbidity and UC activity was a primary endpoint, while EIMs treatment influence over the course of UC in the active phase was a secondary endpoint. For this purpose, the treatment provided to patients when EIM and UC took place simultaneously was analyzed. Likewise, the recovery time of the underlying UC in EIMs was recorded (in months).
Fig. 2.
UC immunological comorbidity
This figure showed how UC and UC immunological comorbidity activity in a patient were followed over time.
CAMD: Common Activity Manifestation Disease
UC: ulcerative colitis
Pearson’s correlation coefficient was used to correlate the retrieved data. Statistical significance was set at P<0.05.32 Microsoft Excel 2016 (v16.0) was used to process the collected data.
RESULTS
One-hundred and five records of patients diagnosed with UC and other comorbidities were retrieved. The patients had a mean age of 37±14 years, a median duration of illness of 8 years, and total colitis was five times higher than that of left-side colitis (Table 2). From these patients, 176 comorbidities were followed: 24% gastroenterological comorbidities; 13% endocrinological comorbidities; 10% rheumatological comorbidities; and 10% cardiological comorbidities (Table 3). Among these, the percentage of comorbidities and UC in the active phase was 43.7%. In that group, dermatological comorbidities accounted for 70%, rheumatological comorbidities accounted for 82.4%, and gastroenterological comorbidities accounted for approximately 65.1%. The comorbidities evaluated in this study were: peripheral arthritis, AS, RA, PG, atopic dermatitis, and PSC.2,24,33
Table 2.
Main characteristics of patients analyzed
| Mean age | 34±14 years old (21–69) |
| Duration of illness (median) | 8 years (2–33) |
| Disease type | Total colitis: left side colitis = 5:1 |
| Lichtiger index | 4.2±1.6 |
| Serum CRP value | 0.3±11 mg/dL |
CRP: C-reactive protein
Table 3.
Characteristics from all EIM, with emphasis on percentage UC in active phase
| EIM | Age (mean) |
Sex, male
n (%) |
UC diagnosis
(median age) |
UC in active phase
(%) |
| Pneumological | 54 (36–86) | 11 (78.6) | 41 (20–79) | 33.3 |
| Hematological | 49 (32–89) | 7 (46.7) | 35 (4–79) | 40.0 |
| Cardiological | 52 (22–73) | 8 (47.1) | 38 (11–65) | 41.1 |
| Nephrological | 55 (28–86) | 5 (71.4) | 41 (24–59) | 57.1 |
| Gynecological | 52 (33–79) | 0 (0.0) | 33 (6–18) | 0 |
| Ophthalmological | 59 (46–69) | 2 (40.0) | 49 (46–58) | 40.0 |
| Dermatological | 47 (30–70) | 4 (40.0) | 35 (17–53) | 70.0 |
| Rheumatological | 59 (32–89) | 5 (29.4) | 40 (17–67) | 82.4 |
| Gastroenterological | 58 (31–86) | 28 (65.1) | 37 (17–59) | 65.1 |
| Neurological | 62 (38–82) | 4 (66.7) | 44 (22–66) | 33.3 |
| Surgical | 61 (47–77) | 3 (42.9) | 41 (27–57) | 14.3 |
| Endocrinological | 62 (42–84) | 11 (47.8) | 44 (21–67) | 52.2 |
| Others | 59 (35–84) | 2 (33.3) | 45 (18–67) | 38.4 |
| Total | 56 (22–89) | 94 (53.4) | 39 (4–79) | 43.7% |
EIM: extraintestinal manifestation
UC: ulcerative colitis
The characteristics of patients with immune-mediated inflammatory disease, which include information about treatment of EIMs and UC in the active phase, are shown in Table 4. In that group, 49.4% of the patients were male. In addition, 145 comorbidities (82.3% of the cases) in our study had no immunological association (not EIMs nor IMID). In fact, patients not associated with immunological comorbidities were diagnosed with these comorbidities years before UC onset and did not show symptoms of these comorbidities later on. Furthermore, 31.1% of patients belonging to this group had the same IMID as analyzed in this study (ie, arthritis). Additionally, the median age of this group of patients was 54 years (p < 0.001). Furthermore, the percentages of UC in the active phase among peripheral arthritis, AS, RA, PG, atopic dermatitis, and primary sclerosing cholangitis comorbidities were 87.5%, 50%, 100%, 75%, 83.3%, and 85.7%, respectively. Likewise, UC recovery time and UC recovered percentage for each EIM were: peripheral arthritis (51.8 months [1–132], 50%); AS (168 months [24–168], 0%); RA (57.4 months [0.53–168], 0%); PG (0.6 months [0.53–0.67], 50%); atopic dermatitis (15.3 months [1–36], 66.7%); and primary sclerosing cholangitis (128 months [60–168], 57.1%).
Table 4.
Characteristics of IMID
| IMID | Age |
Sex, male
n (%) |
Family background | Usual treatment while UC in active phase | UC in active phase (%) | UC recovered (%) |
| Arthritis | 56 (32–78) | 7 (87.5) | Arthritis, Dermatomyositis | Tacrolimus 5 mg, Infliximab 5 mg/kg + Mesalazine 400 mg/day | 87.5 | 50 |
| Ankylosing spondylitis | 54 (43–65) | 2 (100) | None | Infliximab 5 mg/kg + Mesalazine 400 mg/day | 50 | 0 |
| Rheumatoid arthritis | 75 (53–84) | 1 (25) | None | Mesalazine 250 mg 3/day | 100 | 0 |
| Pyoderma gangrenosum | 42 (30–57) | 3 (75) | None | Mesalazine 250 mg/ 3day, Prednisolone 5 mg 2/day | 75 | 50 |
| Dermatitis | 52 (43–60) | 1 (16.7) | Arthritis | Azathioprine, Mesalazine | 83.3 | 66.7 |
| Primary sclerosing cholangitis | 47 (32–68) | 7 (100) | None | Mesalazine 400 mg | 85.7 | 57.1 |
IMID: immune-mediated inflammatory diseases
UC: ulcerative colitis
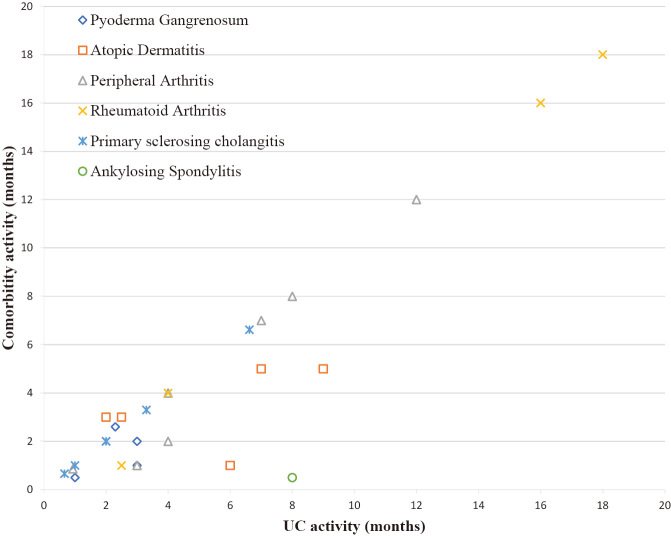
Pearson’s correlation coefficients between UC in the active phase and the comorbidities were: peripheral arthritis (r = 0.97, p < 0.01); RA (r = 0.99, p < 0.01); primary sclerosing cholangitis (r = 0.98, p < 0.01); PG (r = 0.48, p = 0.52); and atopic dermatitis (r = 0.46, p = 0.42) (Fig. 3). Furthermore, UC immunological comorbidity was associated with UC in the active phase (odds ratio [OR], 9.58, 99% CI, 2.52–36.43) (Table 5).34
Fig. 3.
Each comorbidity activity
Graph showed the correlation between UC in the active phase and comorbidities activity over time.
UC: ulcerative colitis
Table 5.
Association between UC in active phase and UC immunological comorbidities (OR, 9.58, 99% CI, 2.52–36.43)
| UC active phase | UC in remission | Total | |
| UC immunological comorbidity | 26 | 5 | 31 |
| Non-UC immunological comorbidity | 51 | 94 | 145 |
| Total | 77 | 99 | 176 |
UC: ulcerative colitis
OR: odds ratio
CI: confidence interval
DISCUSSION
An analysis of comorbidities, especially UC immunological comorbidities, in Japanese patients with UC was performed. EIMs with UC in the active phase accounted for 43.7% of the total EIM, and 26 patients with IMID frequently had an active phase. Comorbidities showing an active manifestation of symptoms and UC in the active phase were significantly correlated in patients with immunological comorbidities, such as peripheral arthritis and RA, as well as in patients with primary sclerosis cholangitis.
Similar studies have been conducted in other parts of the world, such as Switzerland, Canada, and even in the Asian-Pacific area.13,14,33 Comparing our results with those of previous reports, some similarities and differences can be observed. For instance, findings in this cohort showed poor correlation in patients with atopic dermatitis with UC in the active phase. This differs greatly from previous studies,35,36 which marked a correlation between atopic dermatitis as an EIM and UC in the active phase. Similarly, when comparing our results from the PG group, a poor correlation was also shown with UC in the active phase, which also differed from previous reports.37,38 One important point that was different from previous reports is the limited number of patients with both dermatitis and PG in this study. On the other hand, when the arthritis groups were analyzed, there was a substantial correlation with UC in the active phase, and these results are consistent with those of previous studies.14,39 A similar observation could be made when comparing RA with UC in the active phase within patient information. Previous reports showed a similar association.27,40 PSC also showed a substantial correlation with UC in the active phase, which is consistent with previous studies.17,41
Although the underlying pathogenic mechanism involving UC and EIMs remains to be fully understood, there are some hints that may help clarify such mechanisms, and consequently treat these cases adequately.36,42 Previous reports state that in some EIMs, such as PG, symptoms usually manifest at the same time as UC, while in EIMs like PSC and AS, symptoms tend to occur whether UC is in the active phase or not.43 In our study, 75% of PG cases arose while UC was in the active phase, but both diseases overlapped at ±1.04 months. This could be explained by similar pathophysiological pathways shared by some cutaneous manifestations, such as PG and UC.42 High concentrations of adhesion molecules, such as α1β2 (LFA-1) and α4β1 (VLA-4), expressed by circulating gut-activated lymphocytes, are eventually recruited to endothelial ligands such as ICAM-1 and VCAM-1, leading to the development of EIM in UC in the active phase.43 Furthermore, there have been some common links between EIMs and UC at the genetic level. For example, HLA-DRB1*0103, HLA-B27, and HLA-B58 alleles are associated with EIM in patients with UC.44 More specifically, the HLA-class II allele DRB1*0103 is linked with type 1 peripheral arthritis, whereas HLA-B27 is linked with AS. This might explain the shared pathways with UC, with T-cell apoptosis being the most predominant.44 In our study, 78.6% of arthritis patients with UC had type 1 peripheral arthritis, suggesting that HLA-DRB1*0103 might be the allele present in this cohort. Likewise, some studies state that long-lived memory T lymphocytes activated in the gut may also recirculate through the liver, triggering inflammation under special conditions.43 This may have some impact on EIM like PSC, in which expression of potential addressins like VAP-1 and especially MAdCAM-1, would appear to be essential in the recirculation of long-lived memory T lymphocytes between the gut and liver.43 In light of this, periodic searches for UC active signs, even after a liver transplant is performed, are important to prevent further complications.17,43 Even though, in this cohort, only 28.6% of PSC patients with UC actively manifested symptoms even after liver transplant, further research would be necessary to gain a deeper understanding of this topic.
As established, disease severity is important to provide suitable treatment.5 Aminosalicylates are used to treat mild to moderate UC, topical and systemic steroids for UC flares, and immunosuppressants and biological drugs for moderate to severe UC.6,45 In general terms, in EIMs cases, adequate treatment of the underlying UC during the active phase usually induces EIM remission.24,46 Nevertheless, in some cases the EIM course is independent from UC activity.47,48 For example, previous studies have established that antibodies to tumor necrosis factor alpha (anti-TNFα) and corticosteroids, which have a positive effect on UC in the active phase (moderate to severe degree), have a similar effect when IMIDs, such as arthritis, RA, and AS, symptoms manifest.13,24,49,50 In peripheral arthritis cases from our sample, when UC was in the active phase, anti-TNFα (infliximab) and 5-ASA (Mesalazine) were used, with positive results for both UC and EIMs. Similarly, AS was handled while UC was in the active phase; however, given that few patients with AS as EIM were recruited, a broader sample would be needed to make further assumptions. In RA cases, 5-ASA (Mesalazine) was mainly used as maintenance therapy, and anti-TNFα (infliximab) was added when UC was in the active phase, and anti-TNFα stopped when UC activity was controlled.
Regarding management of PG and UC in the active phase, current information is contradictory. One study has reported that treatment of UC does not necessarily work on underlying EIM (PG),48 while others have reported good results in similar circumstances.51,52 Nevertheless, most health centers tend to use 5-ASA in combination with corticosteroids in these cases, given their positive impact on both UC and PG.51 PG patients from our sample were administered 5-ASA and corticosteroids (prednisolone) when UC was in the active phase, showing good recovery. Dermatitis as an EIM while UC was in the active phase in this study was managed with the administration of 5-ASA (Mesalazine). In some patients, immunosuppressors, such as azathioprine, were used, showing clinical remission. Current information suggests that using anti-TNFα would lead to a paradoxical effect, which would be counterproductive when treating UC, while underlying dermatitis takes place.36 Epidermal permeability membrane dysfunction, increased susceptibility due to dysbiosis, interferon-γ secreting TH1 cells, TH17 cells (interleukin 17A and IL-22), plasmacytoid dendritic cells (interferon-α), and keratinocytes (IL-36 and IL-17C) have been proposed to explain this phenomenon, but this topic remains to be fully understood.36 Therefore, it is important to look after adverse mucocutaneous reactions, such as eczematous and psoriasis-like reactions, and even some life-threatening disorders such as Stevens Johnson syndrome and urticaria-angioedema, when prescribing anti-TNF-α, especially when there is a family history of dermatologic comorbidities.36 Previous literature shows that PSC symptoms while UC is in the active phase tend to have a milder clinical course after surgical treatment (liver transplant).17,53 In our cohort, PSC while UC was active had a similar pattern to previous studies. Given that cholangiocarcinoma is a possible outcome in patients with PSC and UC,13 early diagnosis of cholangiocarcinoma (ie, biopsy analysis with signs of adenocarcinoma or mucinous carcinoma) is vital to prevent fatal outcomes in PSC.54
There were some limitations to this study. First, the number of patients with both UC and EIMs followed in this study was limited. This was especially seen in dermatologic comorbidities (atopic dermatitis and PG). Second, while most of the data were collected from electronic medical records, some minor details were not registered explicitly.
In conclusion, this analysis corroborates, to some extent, what previous studies from other parts of the world have stated. Furthermore, it suggests the importance of having full comprehension of how immunologic comorbidities affect the natural development of UC, which is of vital importance to prevent further UC complications and properly adjust its treatment.
AUTHOR CONTRIBUTIONS
Conception and design: Miguel Ricardo Rodríguez Meza, Nakamura M, Yamamura T, Furukawa K. Analysis and interpretation of the data: Miguel Ricardo Rodríguez Meza, Nakamura M, Yamamura T, Kakushima N, Maeda K, Ishikawa E, Ishikawa T, Mizutani Y, Ohno E, Sawada T; drafting of the article: Miguel Ricardo Rodríguez Meza, Nakamura M, Yamamura T; critical revision of the article for important intellectual content: Iida T, Honda T, Kawashima H; and final approval of the article: Ishigami M.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
FUNDING STATEMENT
We have no funding to declare with this study.
Abbreviations
- IBD
inflammatory bowel disease
- CD
Crohn’s disease
- UC
ulcerative colitis
- EIMs
extra-intestinal manifestations
- IMID
immune-mediated inflammatory disease
- AS
ankylosing spondylitis
- PSC
primary sclerosing cholangitis
- RA
rheumatoid arthritis
- PG
pyoderma gangrenosum
- CAMD
Common Activity Manifestation Duration
REFERENCES
- 1.Gajendran M, Loganathan P, Jimenez G, et al. A comprehensive review and update on ulcerative colitis. Dis Mon. 2019;65(12):100851. doi: 10.1016/j.disamonth.2019.02.004. [DOI] [PubMed]
- 2.Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–670. doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed]
- 3.Conrad K, Roggenbuck D, Laass MW. Diagnosis and classification of ulcerative colitis. Autoimmun Reviews. 2014;13(4):463–466. doi: 10.1016/j.autrev.2014.01.028. [DOI] [PubMed]
- 4.Head KA, Jurenka JS. Inflammatory bowel disease part 1: Ulcerative colitispathophysiology and conventional and alternative treatment options. Altern Med Rev. 2003;8(3):247–283. [PubMed]
- 5.Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014;89(11):1553–1563. doi: 10.1016/j.mayocp.2014.07.002. [DOI] [PubMed]
- 6.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed]
- 7.Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14(12):1660–1666. doi: 10.1002/ibd.20520. [DOI] [PMC free article] [PubMed]
- 8.Hirai F, Matsui T, Aoyagi K, et al. Validity of activity indices in ulcerative colitis: comparison of clinical and endoscopic indices. Dig Endosc. 2010;22(1):39–44. doi: 10.1111/j.1443-1661.2009.00916.x. [DOI] [PubMed]
- 9.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43(1):29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed]
- 10.Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20(8): 970–979. doi: 10.1038/s41590-019-0415-0. [DOI] [PubMed]
- 11.Marineaţă A, Rezuş E, Mihai C, Prelipcean CC. Extra intestinal manifestations and complications in inflammatory bowel disease. Rev Med Chir Soc Med Nat Iasi. 2014;118(2):279–288. [PubMed]
- 12.Hernández-Tejero M, Granja Navacerrada A, Bernal Checa P, et al. Prevalence, risk factors and response to treatment of extra-intestinal manifestations in patients with inflammatory bowel disease. Rev Esp Enferm Dig. 2017;109(9): 627–633. doi: 10.17235/reed.2017.4845/2017. [DOI] [PubMed]
- 13.Bernstein CN, Benchimol EI, Bitton A, et al. The impact of inflammatory bowel disease in Canada 2018: Extra-intestinal diseases in IBD. J Can Assoc Gastroenterol. 2019;2(Suppl 1):S73–S80. doi: 10.1093/jcag/gwy053. [DOI] [PMC free article] [PubMed]
- 14.Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106(1):110–119. doi: 10.1038/ajg.2010.343. [DOI] [PubMed]
- 15.Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14(2):111–119. doi: 10.5217/ir.2016.14.2.111. [DOI] [PMC free article] [PubMed]
- 16.Nambu R, Hagiwara SI, Kubota M, Kagimoto S. Difference between early onset and late-onset pediatric ulcerative colitis. Pediatr Int. 2016;58(9):862–866. doi: 10.1111/ped.12935. [DOI] [PubMed]
- 17.Kumagai J, Taida T, Ogasawara S, et al. Clinical characteristics and outcomes of primary sclerosing cholangitis and ulcerative colitis in Japanese patients. PLoS One. 2018;13(12):e0209352. doi: 10.1371/journal.pone.0209352. [DOI] [PMC free article] [PubMed]
- 18.Walsh AJ, Ghosh A, Brain AO, et al. Comparing disease activity indices in ulcerative colitis. J Crohns Colitis. 2014;8(4):318–325. doi: 10.1016/j.crohns.2013.09.010. [DOI] [PubMed]
- 19.Panés J, Su C, Bushmakin AG, Cappelleri JC, Mamolo C, Healey P. Randomized trial of tofacitinib in active ulcerative colitis: analysis of efficacy based on patient-reported outcomes. BMC Gastroenterol. 2015;15:14. doi: 10.1186/s12876-015-0239-9. [DOI] [PMC free article] [PubMed]
- 20.Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: A study of 700 patients. Medicine (Baltimore). 1976;55(5):401–412. doi: 10.1097/00005792-197609000-00004. [DOI] [PubMed]
- 21.Blaney C, Sommer J, El-Gabalawy R, et al. Incidence and temporal trends of co-occurring personality disorder diagnoses in immune-mediated inflammatory diseases. Epidemiol Psychiatr Sci. 2020;29:e84. doi: 10.1017/S2045796019000854. [DOI] [PMC free article] [PubMed]
- 22.Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis. 2018;77(2):175–187. doi: 10.1136/annrheumdis-2017-211555. [DOI] [PubMed]
- 23.Isaacs JD, Cutolo M, Keystone EC, Park W, Braun J. Biosimilars in immune-mediated inflammatory diseases: initial lessons from the first approved biosimilar anti-tumour necrosis factor monoclonal antibody. J Intern Med. 2016;279(1):41–59. doi: 10.1111/joim.12432. [DOI] [PubMed]
- 24.Harbord M, Annese V, Vavricka SR, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2016;10(3):239–254. doi: 10.1093/ecco-jcc/jjv213. [DOI] [PMC free article] [PubMed]
- 25.Jockenhöfer F, Wollina U, Salva KA, Benson S, Dissemond J. The PARACELSUS score: a novel diagnostic tool for pyoderma gangrenosum. Br J Dermatol. 2019;180(3):615–620. doi: 10.1111/bjd.16401. [DOI] [PubMed]
- 26.Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157(4):645–648. doi: 10.1111/j.1365-2133.2007.08112.x. [DOI] [PubMed]
- 27.Fricker ZP, Lichtenstein DR. Primary sclerosing cholangitis: a concise review of diagnosis and management. Dig Dis Sci. 2019;64(3):632–642. doi: 10.1007/s10620-019-05484-y. [DOI] [PubMed]
- 28.Pujalte GG, Albano-Aluquin SA. Differential diagnosis of polyarticular arthritis. Am Fam Physician. 2015;92(1): 35–41. [PubMed]
- 29.Wasserman AM. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2011;84(11):1245–1252. [PubMed]
- 30.van Riel PL, Renskers L. The Disease Activity Score (DAS) and the Disease Activity Score using 28 joints counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(5 Suppl 101):S40–S44. [PubMed]
- 31.Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res (Hoboken). 2011:63 Suppl 11:S47–S58. doi: 10.1002/acr.20575. [DOI] [PubMed]
- 32.Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91–93. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed]
- 33.Ooi CJ, Fock KM, Makharia GK, et al. The Asia-Pacific consensus on ulcerative colitis. J Gastroenterol Hepatol. 2010;25(3):453–468. doi: 10.1111/j.1440-1746.2010.06241.x. [DOI] [PubMed]
- 34.Szumilas M. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry. 2010;19(3):227–229. [PMC free article] [PubMed]
- 35.Nader A, Stodtmann S, Friedel A, Mohamed MF, Othman AA. Pharmacokinetics of upadacitinib in healthy subjects and subjects with rheumatoid arthritis, Crohn’s disease, ulcerative colitis, or atopic dermatitis: Population analyses of phase 1 and 2 clinical trials. J Clin Pharmacol. 2020;60(4):528–539. doi: 10.1002/jcph.1550. [DOI] [PubMed]
- 36.Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10(2):364. doi: 10.3390/jcm10020364. [DOI] [PMC free article] [PubMed]
- 37.Shibuya T, Haga K, Saeki M, et al. Pyoderma gangrenosum in an ulcerative colitis patient during treatment with vedolizumab responded favorably to adsorptive granulocyte and monocyte apheresis. J Clin Apher. 2020;35(5):488–492. doi: 10.1002/jca.21821. [DOI] [PubMed]
- 38.Goreti Catorze M, Pereira F, Fonseca F, Morbey A, Assis Pacheco F. Pyoderma gangrenosum associated with sclerosing cholangitis, type 1 diabetes mellitus and ulcerative colitis. J Eur Acad Dermatol Venereol. 2001;15(3):257–259. doi: 10.1046/j.1468-3083.2001.00260.x. [DOI] [PubMed]
- 39.Sato T, Konno J, Sekiguchi A, et al. Long-lasting immunosuppressive effects of tacrolimus-loaded micelle NK61060 in preclinical arthritis and colitis models. Ther Deliv. 2018;9(10):711–729. doi: 10.4155/tde-2018-0044. [DOI] [PubMed]
- 40.Rubin DT, Mittal M, Davis M, Johnson S, Chao J, Skup M. Impact of a patient support program on patient adherence to adalimumab and direct medical costs in Crohn’s disease, ulcerative colitis, rheumatoid arthritis, psoriasis, psoriatic arthritis, and ankylosing spondylitis. J Manag Care Spec Pharm. 2017;23(8):859–867. doi: 10.18553/jmcp.2017.16272. [DOI] [PMC free article] [PubMed]
- 41.Tanaka A, Mertens JC. Ulcerative colitis with and without primary sclerosing cholangitis: Two different diseases? Inflamm Intest Dis. 2016;1(1):9–14. doi: 10.1159/000445259. [DOI] [PMC free article] [PubMed]
- 42.Marotto D, Atzeni F, Ardizzone S, Monteleone G, Giorgi V, Sarzi-Puttini P. Extra-intestinal manifestations of inflammatory bowel diseases. Pharmacol Res. 2020;161:105206. doi: 10.1016/j.phrs.2020.105206. [DOI] [PubMed]
- 43.Eksteen B, Miles AE, Grant AJ, Adams DH. Lymphocyte homing in the pathogenesis of extra-intestinal manifestations of inflammatory bowel disease. Clin Med (Lond). 2004;4(2):173–180. doi: 10.7861/clinmedicine.4-2-173. [DOI] [PMC free article] [PubMed]
- 44.Perez-Alamino R, Maldonado-Ficco H, Maldonado-Cocco JA. Rheumatic manifestations in inflammatory bowel diseases: a link between GI and rheumatology. Clin Rheumatol. 2016;35(2):291–296. doi: 10.1007/s10067-015-3116-6. [DOI] [PubMed]
- 45.Yamamoto-Furusho JK, Gutiérrez-Grobe Y, López-Gómez JG, Bosques-Padilla F, Rocha-Ramírez JL; Grupo del Consenso Mexicano de Colitis Ulcerosa Crónica Idiopática. The Mexican consensus on the diagnosis and treatment of ulcerative colitis [in Spanish; English]. Rev Gastroenterol Mex (Engl Ed). 2018;83(2):144–167. doi: 10.1016/j.rgmx.2017.08.006. [DOI] [PubMed]
- 46.Paredes JM, Barrachina MM, Román J, Moreno-Osset E. Joint disease in inflammatory bowel disease [in Spanish]. Gastroenterol Hepatol. 2005;28(4):240–249. doi: 10.1157/13073095. [DOI] [PubMed]
- 47.Atzeni F, Ardizzone S, Bertani L, Antivalle M, Batticciotto A, Sarzi-Puttini P. Combined therapeutic approach: inflammatory bowel diseases and peripheral or axial arthritis. World J Gastroenterol. 2009;15(20):2469–2471. doi: 10.3748/wjg.15.2469. [DOI] [PMC free article] [PubMed]
- 48.Menachem Y, Gotsman I. Clinical manifestations of pyoderma gangrenosum associated with inflammatory bowel disease. Isr Med Assoc J. 2004;6(2):88–90. [PubMed]
- 49.Peluso R, Manguso F, Vitiello M, Iervolino S, Di Minno MN. Management of arthropathy in inflammatory bowel diseases. Ther Adv Chronic Dis. 2005;6(2):65–77. doi: 10.1177/2040622314563929. [DOI] [PMC free article] [PubMed]
- 50.Hedin CRH, Vavricka SR, Stagg AJ, et al. The pathogenesis of extraintestinal manifestations: Implications for IBD research, diagnosis, and therapy. J Crohns Colitis. 2019;13(5):541–554. doi: 10.1093/ecco-jcc/jjy191. [DOI] [PubMed]
- 51.Chen W, Xiang L, Li L. Therapeutic efficacy of the combination therapy of corticosteroids and 5-aminosalicylic acid for treatment of pyoderma gangrenosum with ulcerative colitis. Indian J Dermatol. 2020;65(1):38–41. doi: 10.4103/ijd.IJD_505_18. [DOI] [PMC free article] [PubMed]
- 52.Arivarasan K, Bhardwaj V, Sud S, Sachdeva S, Puri AS. Biologics for the treatment of pyoderma gangrenosum in ulcerative colitis. Intest Res. 2016;14(4):365–368. doi: 10.5217/ir.2016.14.4.365. [DOI] [PMC free article] [PubMed]
- 53.Aranake-Chrisinger J, Dassopoulos T, Yan Y, Nalbantoglu I. Primary sclerosing cholangitis associated colitis: Characterization of clinical, histologic features, and their associations with liver transplantation. World J Gastroenterol. 2020;26(28):4126–4139. doi: 10.3748/wjg.v26.i28.4126. [DOI] [PMC free article] [PubMed]
- 54.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1219. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed]