Fig. 1.
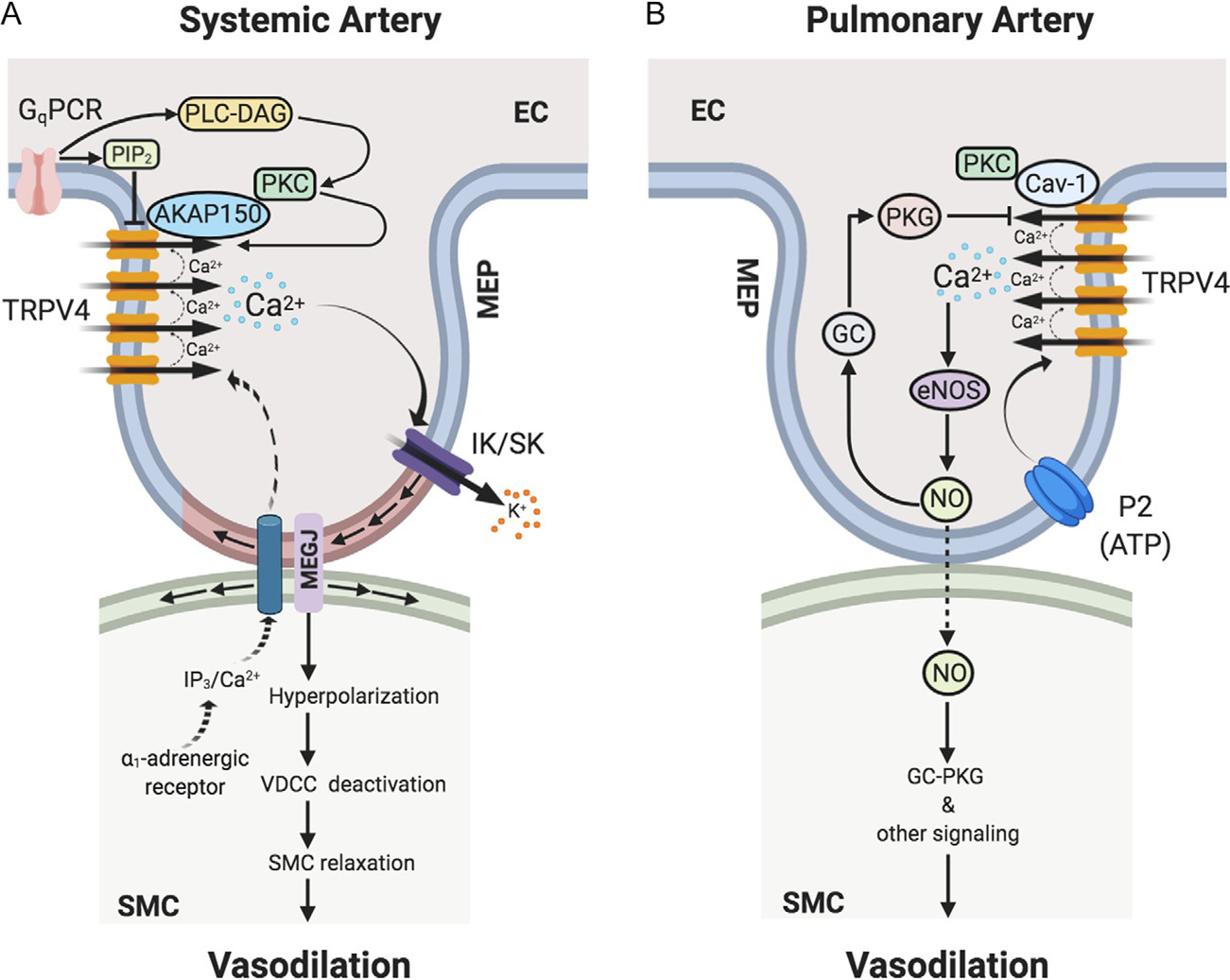
TRPV4 channel-dependent Ca2+ signaling at endothelial projections to smooth muscle cells or myoendothelial projections (MEPs) regulates vasodilation in (A) systemic and (B) pulmonary arteries. (A) In systemic artery, Gq protein-coupled receptor (GqPCR) stimulation activates phospholipase C (PLC)-diacylglycerol (DAG)-protein kinase C (PKC) signaling. PKC anchoring by AKAP150 enhances the activity and coupled openings of TRPV4 channels. In capillaries, GqPCR signaling can lower the levels of PIP2, thereby increasing TRPV4 channel activity. Ca2+ influx through TRPV4 channels selectively activates IK and SK channels at MEPs, thereby hyperpolarizing endothelial cell (EC) and smooth muscle cell (SMC) membranes. SMC membrane hyperpolarization leads to deactivation of voltage-dependent Ca2+ channels (VDCCs), thus relaxing SMCs and causing vasodilation. In response to SMC α1-adrenergic stimulation, IP3/Ca2+ from SMCs can cross MEGJs to enter EC layer to limit vasoconstriction via endothelial mechanisms. (B) In pulmonary artery, Ca2+ influx through TRPV4 channels selectively activates endothelial nitric oxide synthase (eNOS) to cause NO release and vasodilation. P2 purinergic receptor agonist ATP is an endogenous activator of the TRPV4-eNOS signaling. Endothelial caveolin-1 (Cav-1) enhances endothelial TRPV4 channel activity. Endothelium-derived NO subsequently causes the relaxation of SMCs leading to vasodilation via SMC guanylyl cyclase (GC)—protein kinase G (PKG)-dependent and -independent signaling mechanisms. NO also elicits activation of endothelial GC-PKG signaling, which lowers the coupled openings of TRPV4 channels, thereby limiting Ca2+ influx through TRPV4 channels. AKAP150, a-kinase anchoring protein 150; MEP, MEGJ, myoendothelial gap junction; IK/SK, intermediate/small-conductance Ca2+-activated K+ channels; SMC, smooth muscle cell; IP3, inositol trisphosphate.
