Abstract
The effect of phase variation of lipopolysaccharide (LPS) structure on the susceptibility of Haemophilus influenzae to complement-dependent killing by normal human sera and normal rat sera has been described previously. The phase-variable structure phosphorylcholine (ChoP) confers susceptibility to human serum, since ChoP on the bacterial cell surface binds to serum C-reactive protein and activates complement. In contrast, expression of galα1,4gal, a second phase-variable epitope that is also found on human glycoconjugates, confers resistance to human serum. We studied the role of phase variation of these structures in the susceptibilities of H. influenzae KW20 (Rd) and a clinical isolate of nontypeable H. influenzae to killing by rabbit sera, which often possess naturally acquired complement-dependent bactericidal activity for unencapsulated H. influenzae. Expression of ChoP increased the resistance of strain KW20 to killing by bactericidal rabbit sera. In contrast, the serum resistance of a clinical isolate, H233, was unaffected by ChoP expression but was reduced by galα1,4gal expression. The rabbit sera with bactericidal activity (but not the nonbactericidal sera) all contained immunoglobulin M (IgM) antibodies able to bind to the surface of H. influenzae bacteria, as detected by flow cytometry, and contained IgM antibodies to LPS purified from strain KW20. Preincubation of sera with LPS reduced their bactericidal activity. Bactericidal activity was recovered quantitatively in an IgM-enriched fraction of sera. It is concluded that naturally occuring bactericidal activity for unencapsulated H. influenzae is largely due to IgM antibodies directed against phase-variable structures of the LPS.
It has long been known that normal human sera and sera from infant or adult rats are often bactericidal for unencapsulated Haemophilus influenzae and for type b H. influenzae. Further, any given isolate of H. influenzae undergoes high-frequency switching between a serum-resistant and a serum-sensitive state (17, 18). The molecular basis of this phenotypic variation has been the subject of extensive study over the past several years. It has been shown to be the result, at least in part, of phase variation in the expression of surface-exposed lipopolysaccharide (LPS) antigenic structures. These include phosphorylcholine (ChoP) and galα1,4gal, both of which mimic structures found on the surface of host cells (18, 19). Substitution of LPS by ChoP is mediated by the lic1 locus of H. influenzae (17). Expression of galα1,4gal requires the lic2 and lgtC loci (8, 13). Expression of ChoP increases the sensitivity of H. influenzae isolates to normal human sera, since human serum contains C-reactive protein, which binds to the ChoP, activating complement and killing the bacteria (19). In contrast, sera from normal rats contain very low levels of C-reactive protein, and expression of ChoP increases the resistance of H. influenzae isolates to the bactericidal activity of rat sera (18). Expression of galα1,4gal increases sensitivity to rat sera, but not to human sera (18). This suggests that rats, but not humans, often have naturally occuring antibodies to this epitope, which is also found on human glycolipids.
Like humans and rats, laboratory rabbits often have naturally occuring serum bactericidal activity for H. influenzae. In this study, we report that this activity is mediated by immunoglobulin M (IgM) antibodies directed against phase-variable LPS structures. We were able to determine, in part, the target of the bactericidal antibodies. The epitopes targeted include galα1,4gal, the structure which confers serum resistance in human serum, but not ChoP, which appears to mask bactericidal epitopes in some strains.
MATERIALS AND METHODS
Bactericidal assay.
H. influenzae strains used in this study are listed in Table 1. The procedure for the bactericidal assay was modified from that described by Shurin et al. (16). Except as indicated (Table 2), bactericidal assays were carried out by using strain KW20 from the strain collection at MedImmune (KW20-MI) (see Table 1), grown to mid-log phase in brain heart infusion medium (Difco) supplemented with 10 μg of nicotinamide adenine dinucleotide per ml and either Levinthal's supplement (added at 10%, vol/vol [1]) or 10 μg of hemin per ml. Reactions were carried out in 96-well polystyrene plates with round-bottomed wells. Each reaction (0.1-ml final volume) contained 10 μl of complement (3- to 4-week-old rabbit complement; Pel-Freez, Brown Deer, Wis.), approximately 200 bacteria, and varying dilutions of test serum. The diluent was Gey's balanced salt solution (Sigma Chemical Co., St. Louis, Mo.). The reactions were incubated for 30 min at 37°C on a rocking platform. The plate was then placed on ice, and 20 μl from each fraction was spotted onto a GC-hemin plate, (prepared from GC II agar [BBL Microbiology Systems] supplemented with Isovitalex [1%, vol/vol, BBL] and 10 μg of hemin per ml). The agar plates were allowed to dry and were then incubated overnight at 37°C in 5% CO2. The endogenous complement in test sera was inactivated by heating for 30 min at 56°C, and the sera were diluted to final concentrations ranging from 1:20 to 1:1,280. All reactions were carried out in duplicate, and the colony counts were averaged. The bactericidal titer of a serum was defined as the highest dilution resulting in 50%-or-greater reduction in viable bacteria, compared to control wells in which bacteria were incubated with complement but no serum. Each lot of complement purchased was prescreened to ensure that it would support the killing of H. influenzae KW20 by antiserum to outer membrane and that killing in the absence of added serum was minimal.
TABLE 1.
H. influenzae isolates used in this study
| Strain | Relevant characteristics | Reference |
|---|---|---|
| KW20-MI | Isolate of KW20 in MedImmune strain collection | 5, 21 |
| KW20-H3 | Isolate of KW20 in Weiser strain collection | 5, 21 |
| H446 | lic1 knockout of KW20-H3, ChoP not expressed | 12 |
| H491 | lic1 constitutive “on” mutant of KW20-H3, ChoP expressed constitutively | 12 |
| H418 | HAS+ 4C4− variant of nontypeable clinical isolate H233 | 18 |
| H419 | HAS− 4C4− variant of nontypeable clinical isolate H233 | 18 |
| H420 | HAS− 4C4+ variant of nontypeable clinical isolate H233 | 18 |
TABLE 2.
Effect of variations in LPS structure on sensitivity to bactericidal activity of rabbit sera
| Variation | Isolate | Genotype | Phenotype (% of colonies reactive with:)
|
Bactericidal titer of rabbit serum:
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HASa | 4C4 | ME46b | ME48 | ME53 | ME56 | ME60 | ME88 | |||
| Mutation of lic1 locus | KW20-MI | Wild type | 13.8 | NDc | >1,280d | 320 | 160 | <20 | <20 | >1,280 |
| KW20-H3 | Wild type | 96.5 | ND | 160 | 80 | 80 | <20 | <20 | 160 | |
| H446 | lic1 | 0 | ND | >1,280 | 1,280 | 640 | <20 | <20 | >1,280 | |
| H491 | lic1(Con) | 100 | ND | 20 | 20 | <20 | <20 | <20 | 20 | |
| Phenotypic variation of ChoP and galα1,4gal | KW20-MI | 2.9 | <1 | >1,280 | 1,280 | >1,280 | <20 | <20 | >1,280 | |
| H418 | 95.5 | <1 | 40 | 40 | 40 | <20 | <20 | <20 | ||
| H419 | 2.5 | <1 | 80 | 20 | 40 | <20 | <20 | 40 | ||
| H420 | 2.9 | 92.2 | >1,280 | >1,280 | >1,280 | <20 | <20 | >1,280 | ||
The expression of the phase-variable LPS epitopes ChoP and galα1,4gal was determined for each culture by colony immunoblotting by using monoclonal antibodies HAS (for ChoP) and 4C4 I(for galα1,4gal), as previously described previously (18).
Rabbits were immunized with proteins cloned from H. influenzae KW20 (5). The predicted sequences of these proteins are available from the TIGR database (www.tigr.org). Serum ME46, locus HI 0541; ME48, HI 0947; ME53, HI 1119; ME56, HI 1373, ME60, HI 1251. Serum ME88 is from a rabbit immunized with OM prepared from KW20.
ND, not determined (monoclonal antibody 4C4 does not bind to KW20 [15]).
Bactericidal titer.
Flow cytometric detection of antibody bound to bacterial cells.
Bacteria were grown to mid-log phase as described above, then washed in Hanks' balanced salt solution (Life Technologies, Grand Island, N.Y.) containing 5% fetal bovine serum and were suspended in the same buffer to approximately 5 × 108 CFU/ml. A 25-μl volume of bacterial suspension was added to 25 μl of diluted serum (final serum dilution, 1:20 or 1:50). Bacteria were incubated with serum for 1 h at 4°C and were then washed and suspended in 50 μl of phycoerythrin-conjugated goat anti-rabbit Ig (α-Ig) or anti-rabbit IgM (α-IgM) (Southern Biotechnology Associates, Inc., Birmingham, Ala.) (final antibody concentration of 5 μg/ml). After 1 h of incubation at 4°C in the dark, the bacteria were washed and resuspended in 1 ml of Hanks' balanced salt solution–5% fetal bovine serum for analysis by flow cytometry. Samples were acquired and analyzed on a FacStar Plus flow cytometer by using CellQuest software from Becton Dickinson Immunocytometry Systems (San Jose, Calif.). Logarithmic amplification of all parameters was used with a side scatter threshold. An electronic gate was used to limit acquisition and analysis to the singlet bacteria population. Ten thousand events per sample were collected and analyzed for the detection of bound antibodies as indicated by a shift in the fluorescent signal above background.
Enzyme-linked immunosorbent assay (ELISA).
Anti-LPS antibodies were measured as follows: a suspension of LPS (purified from strain KW20 by hot phenol-water extraction as described [4]) was diluted to 10 μg per ml in Dulbecco's phosphate-buffered saline (PBS; Hyclone, Logan, Utah) containing 10 mM MgCl2 and was used to coat Immulon 2HB plates (Dynex Technologies, Inc., Chantilly, Va.) (100 μl per well). Plates were incubated overnight at 4°C and then wells were filled with blocking solution consisting of 5% bovine serum albumin fraction V (Sigma) in PBS containing 0.2% Tween 20, and the plates were incubated for 1 h. The wells were washed once with PBS–0.2% Tween 20 using an automatic plate washer (model 96PW; TECAN U.S., Hillsborough, N.C.). A 100-μl aliquot of serum (serially diluted in blocking buffer to a final concentration of from 1:10 to 1:1,280) was added to each well and was incubated for 3 h at room temperature. The plate was washed four times, and 100 μl of secondary antibody (horseradish-peroxidase-conjugated goat anti-rabbit IgG or anti-rabbit IgM, diluted in blocking buffer to 1:5,000; Southern Biotechnology Associates) was added and incubated for 2 h. After washing, bound antibody was detected by using the 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) peroxidase substrate system (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). The optical densities at 405 nm were determined 30 min after addition of substrate by using a Vmax Kinetic Microplate Reader (Molecular Devices Corporation, Menlo Park, Calif.). Each reaction was carried out in duplicate, and the results were averaged. The anti-LPS titer of a serum was defined as the last dilution giving an absorbance greater than twice the background value.
RESULTS AND DISCUSSION
Phase-variable LPS structures affect killing by bactericidal rabbit sera.
In preliminary experiments (data not shown) we tested the bactericidal activity of rabbit antisera to a number of different recombinant H. influenzae proteins that had been cloned from strain KW20 as hexahistidine-tagged proteins, denatured with guanidine, purified by nickel-affinity chromatography, and renatured by dialysis as described (7). Control sera were from rabbits that had been immunized with an outer membrane (OM) preparation of KW20 prepared by Triton X-100 extraction as described by Loeb & Smith (10). Additional control sera were from unimmunized rabbits or rabbits immunized with antigens cloned from Streptococcus pneumoniae or Staphylococcus aureus. We found that over half of the antisera against H. influenzae antigens were bactericidal for H. influenzae KW20, with bactericidal titers as high as 2,560. However, equally high bactericidal titers were seen in many of the antisera to gram-positive antigens, as well as some of the sera from unimmunized rabbits. This suggests that bactericidal activity for unencapsulated H. influenzae may occur naturally in rabbits (as it does in humans and in rats) and is not necessarily the effect of immunization with a given protein antigen. We are therefore unable to draw conclusions about the ability of any of the H. influenzae proteins to elicit bactericidal antibodies.
For evaluation of the role of phase variation in susceptibility to naturally acquired bactericidal activity, we used four rabbit sera with bactericidal activity for H. influenzae KW20 and two sera lacking bactericidal activity. Five of the rabbits had been immunized with recombinant H. influenzae antigens (listed in Table 2), and the sixth was an anti-OM serum. When the six sera were tested against KW20 mutants differing in ChoP expression (Table 2), it was found that the lic1 mutant (lacking ChoP) was the most susceptible to killing by the four bactericidal sera (bactericidal titers were ≥1,280). Strain H491, which expresses ChoP constitutively, was resistant to killing by all six sera, including the anti-OM serum (bactericidal titers were ≤20). Isolates of KW20 from two different laboratory collections were found to differ in the proportion of colonies expressing ChoP, and the strain with higher prevalence of ChoP expression was more resistant to killing. In further experiments (Table 2), we used variants of a clinical isolate, H233, that differed in expression of ChoP and galα1,4gal (detected by monoclonal antibodies HAS and 4C4, respectively) (18). (We were unable to examine the effect of galα1,4gal expression on serum sensitivity of KW20 because the galα1,4gal structure is masked by a terminal N-acetylgalactosamine residue in this strain, preventing the binding of monoclonal antibody 4C4 [15].) For the H233 variants, we found that expression of galα1,4gal increased susceptibility to killing by the four bactericidal sera (titers were >1,280). In the absence of galα1,4gal expression, bacteria were resistant to killing regardless of the presence or absence of ChoP.
These data indicate that the LPS phase-variable structures have an enormous effect on the susceptibility of bacteria to bactericidal antibodies. Even the anti-OM serum, ME88, was unable to kill KW20 if ChoP expression was constitutive. Analysis by Western blotting showed reactivity of serum ME88 with numerous OM proteins of KW20 (data not shown). In strain KW20, the presence of ChoP may act to obscure bactericidal epitopes on the LPS. Because the site of ChoP substitution is not the same in all LPS structures (12), it is perhaps not surprising that for the two strains used in our experiments, ChoP expression had different effects on serum resistance.
Bactericidal activity is the result of IgM antibodies directed against LPS.
The results of the experiments described above suggest that the bactericidal activity we saw in certain rabbit sera was the result of antibody to LPS. However, this is not necessarily the case. As noted above, the bactericidal activity of normal human serum for H. influenzae isolates expressing ChoP is not antibody mediated, but results from C-reactive protein binding to the ChoP on the bacterial surface and activating complement. Further, there is precedent for LPS structure affecting the susceptibility to killing mediated by protein antigens: gonococci with sialylated LPS are resistant not only to killing by normal human sera, but also to killing by antisera to the major OM protein, protein 1 (20).
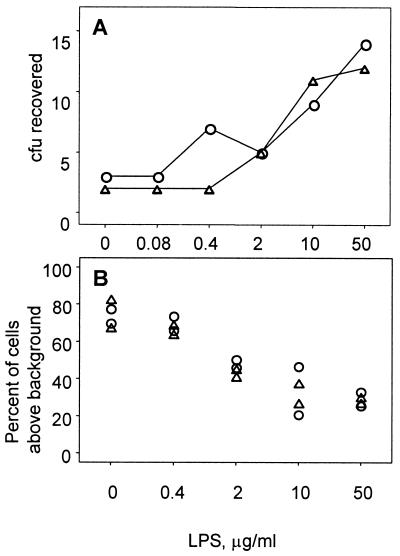
We considered the possibility that the actual target of the bactericidal activity is not LPS, but another antigen whose expression or accessibility is affected by LPS structure. In that case, depletion of anti-LPS antibodies would not affect the bactericidal activity of rabbit sera. In order to evaluate this possibility, we incubated two of the bactericidal sera with varying concentrations of LPS before testing them in a bactericidal assay. We found that preincubation with LPS reduced the bactericidal activity of these sera in a dose-dependent manner (Fig. 1A). Similarly, preincubation with LPS reduced the intensity of binding of IgM to the surface of bacterial cells (Fig. 1B). We were unable to detect binding of IgG to bacterial cells in these sera (data not shown). Both of these experiments are consistent with the conclusion that the bactericidal activity in certain rabbit sera is the result of serum components that recognize epitopes on the purified LPS; the flow cytometry experiments suggest that the serum components are IgM. However, we cannot rule out the possibility that the added LPS bound complement in the bactericidal assay, thereby reducing antibody-dependent killing.
FIG. 1.
Preincubation of sera with LPS reduces both bactericidal activity and binding of IgM to the bacterial cell surface. Rabbit sera ME46 (○) and ME109 (▵) were incubated for 1 h in varying concentrations of LPS before use in the bactericidal assay (panel A) and flow cytometric determination of binding to bacteria (panel B). The LPS concentrations indicated in each panel are the final concentrations during incubations of bacteria with sera. The final serum concentrations were 1:100 for the bactericidal assay and 1:20 for flow cytometry. Serum ME109 was raised against a surface antigen of S. pneumoniae (our unpublished data).
When we measured anti-LPS IgG and IgM antibodies directly in 15 rabbit sera by ELISA, we found that all 15 sera contained IgG antibodies able to bind LPS (titer was ≥320); ELISA titers were unrelated to bactericidal titers. In contrast, of four nonbactericidal sera, none contained IgM reactive with LPS (titer was <20), while all 11 bactericidal sera contained anti-LPS IgM (titer was ≥640). Consistent with this, all bactericidal sera tested contained IgM able to bind to the surface of bacteria, as seen by flow cytometry, while the nonbactericidal sera were all negative in this assay.
The above observations suggested that the bactericidal activity in certain rabbit sera was mediated by IgM antibodies that could be detected by flow cytometry and that those antibodies were directed against the LPS. In order to confirm that bactericidal activity was indeed mediated by IgM, we obtained IgM-enriched fractions from several bactericidal rabbit sera. We took advantage of the fact that IgM is one of the largest molecules in serum, so that passage of serum over an appropriate gel-filtration column will allow recovery of nearly pure IgM in the void volume, while IgG and most other serum proteins are in the included volume (6). Serum (500 μl) was fractionated by using a TSKG3000PWXL column (TosoHaas, Montgomeryville, Pa.), with PBS as the running buffer. The fractions were concentrated to the original serum volumes by ultrafiltration by using a Centricon-30 filter (Millipore Corp., Bedford, Mass.). Bactericidal activity, anti-LPS IgM ELISA reactivity, and IgM bacteria-binding activity were all recovered in the IgM-enriched fraction, while IgG reactivity with the protein immunogen was recovered quantitatively in the IgM-depleted fraction (data not shown). With the exception of the anti-OM serum, no bactericidal activity was recovered in the IgM-depleted fraction. These data suggest that the naturally occuring bactericidal activity in rabbit sera is mediated by IgM, but that immunization with OM produces bactericidal IgG antibodies.
Previous study of the bactericidal activity of normal human and rat sera for H. influenzae has shown the central role of phase-variable LPS structures in controlling resistance to naturally acquired immunity and that whether a given oligosaccharide constituent confers resistance or sensitivity varies from host to host. Our data suggest that the naturally acquired bactericidal antibodies in rabbit sera are similar in specificity to those in rat sera and are different from those in human sera. It has not previously been shown that these antibodies are IgM or that LPS is the dominant target. Rabbits may produce antibodies bactericidal for H. influenzae as a result of exposure to antigens that cross-react with H. influenzae LPS, or these antibodies may be part of the spontaneous repertoire of natural antibodies, which are often IgM (14).
Implications for evaluation of vaccine candidates.
While the attempt to demonstrate bactericidal activity of immune sera is a customary part of the evaluation of H. influenzae vaccine candidates (2, 3), obtaining convincing, reproducible evidence of bactericidal activity is often difficult (11) due to the existence in many laboratory animals of naturally occurring bactericidal activity towards H. influenzae. The level of bactericidal activity in individual animals varies and may increase during the life of the animal, independent of the immunogen. Researchers have tried to minimize the effect of these antibodies. For example, Loeb reported that the sera of rabbits immunized with H. influenzae OM protein a (also known as P1) were bactericidal even after adsorption with capsular polysaccharide and LPS and concluded that antibodies to protein a were bactericidal (9). Our data suggest that the problem of naturally occuring antibodies is compounded by the fact that they are directed against phase-variable epitopes. In practice, adsorption of sera with purified LPS may not remove all the anti-LPS antibodies, because the bacterial culture used for any individual bactericidal assay may express LPS with a different structure from that of the purified LPS.
With increasing knowledge of the chemical structure of LPS from different isolates and the role in serum resistance of epitopes identifiable by monoclonal antibodies, it may be possible to identify phenotypic variants of the target strain that are resistant to naturally occurring bactericidal activity. Such variants might allow better evaluation of the ability of conserved protein vaccine candidates to elicit bactericidal antibodies.
ACKNOWLEDGMENTS
Stephan Goldner, Bucknell University, carried out early experiments characterizing the bactericidal activity of serum fractions and identifying anti-LPS IgM in bactericidal sera. We thank Jon Heinrichs and Scott Koenig for critical review of the manuscript.
This work was supported, in part, by Public Health Service grants AI38436 and AI44231 to J.N.W.
REFERENCES
- 1.Alexander H E, Heidelberger M, Leidy G. The protective or curative element in type b Haemophilus influenzae rabbit serum. Yale J Biol Med. 1944;16:425–434. [PMC free article] [PubMed] [Google Scholar]
- 2.Deich R A, Anilionis A, Fulginiti J, Metcalf B J, Quataert S, Quinn-Dey T, Zlotnick G W, Green B A. Antigenic conservation of the 15,000-Dalton outer membrane lipoprotein PCP of Haemophilus influenzae and biologic activity of anti-PCP antisera. Infect Immun. 1990;58:3388–3393. doi: 10.1128/iai.58.10.3388-3393.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMaria T F, Murwin D M, Leake E R. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun. 1996;64:5187–5192. doi: 10.1128/iai.64.12.5187-5192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erwin A L, Haynes P A, Rice P A, Gotschlich E C. Conservation of the lipooligosaccharide synthesis locus lgt among strains of Neisseria gonorrhoeae: requirement for lgtE in synthesis of the 2C7 epitope and of the beta chain of strain 15253. J Exp Med. 1996;184:1233–1241. doi: 10.1084/jem.184.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 6.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. p. 308. [Google Scholar]
- 7.Heinrichs J H, Gatlin L E, Kunsch C, Choi G H, Hanson M S. Identification and characterization of SirA, an iron-regulated protein from Staphylococcus aureus. Infect Immun. 1999;181:1436–1443. doi: 10.1128/jb.181.5.1436-1443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.High N J, Deadman M E, Moxon E R. The role of a repetitive DNA motif (5′-CAAT-3′) in the variable expression of the Haemophilus influenzae lipopolysaccharide epitope αGal(1-4)βGal. Mol Microbiol. 1993;9:1275–1282. doi: 10.1111/j.1365-2958.1993.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 9.Loeb M R. Protection of infant rats from Haemophilus influenzae type b infection by antiserum to purified outer membrane protein a. Infect Immun. 1987;55:2612–2618. doi: 10.1128/iai.55.11.2612-2618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loeb M R, Smith D H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980;30:709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loosmore S M, Yang Y-P, Coleman D C, Shortreed J M, England D M, Klein M H. Outer membrane protein D15 is conserved among Haemophilus influenzae species and may represent a universal protective antigen against invasive disease. Infect Immun. 1997;65:3701–3707. doi: 10.1128/iai.65.9.3701-3707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lysenko E, Richards J C, Cox A D, Stewart A, Martin A, Kapoor M, Weiser J N. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein mediated killing. Mol Microbiol. 2000;32:234–245. doi: 10.1046/j.1365-2958.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 13.Maskell D J, Szabo M J, Butler P D, Williams A E, Moxon E R. Molecular analysis of a complex locus from Haemophilus influenzae involved in phase-variable lipopolysaccharide biosynthesis. Mol Microbiol. 1991;5:1013–1022. doi: 10.1111/j.1365-2958.1991.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 14.Ochsenbein A F, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel R M. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 15.Risberg A, Masoud H, Martin A, Richards J C, Moxon E R, Schweda E K. Structural analysis of the lipopolysaccharide oligosaccharide epitopes expressed by a capsule-deficient strain of Haemophilus influenzae, Rd. Eur J Biochem. 1999;261:171–180. doi: 10.1046/j.1432-1327.1999.00248.x. [DOI] [PubMed] [Google Scholar]
- 16.Shurin P A, Pelton S I, Tager I B, Kasper D L. Bactericidal antibody and susceptibility to otitis media caused by nontypable strains of Haemophilus influenzae. J Pediatr. 1980;97:364–369. doi: 10.1016/s0022-3476(80)80182-x. [DOI] [PubMed] [Google Scholar]
- 17.Weiser J N. Relationship between colony morphology and the life cycle of Haemophilus influenzae: the contribution of lipopolysaccharide phase variation to pathogenesis. J Infect Dis. 1993;168:672–680. doi: 10.1093/infdis/168.3.672. [DOI] [PubMed] [Google Scholar]
- 18.Weiser J N, Pan N. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol Microbiol. 1998;30:767–775. doi: 10.1046/j.1365-2958.1998.01108.x. [DOI] [PubMed] [Google Scholar]
- 19.Weiser J N, Pan N, McGowan K L, Musher D, Martin A, Richards J. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;184:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wetzler L M, Barry K, Blake M S, Gotschlich E C. Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect Immun. 1992;60:39–43. doi: 10.1128/iai.60.1.39-43.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilcox K W, Smith H O. Isolation and characterization of mutants of Haemophilus influenzae deficient in an adenosine 5′-triphosphate-dependent deoxyribonuclease activity. J Bacteriol. 1975;122:443–453. doi: 10.1128/jb.122.2.443-453.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]