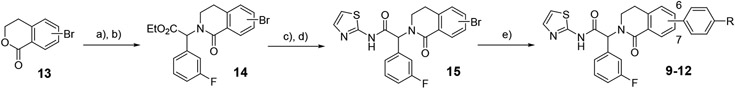
Scheme 1 –
synthesis of dihydroisoquinolinones 9-12. Reagents and conditions: a) PCl5, POCl3, reflux; b) ethyl 2-amino-2-(3-fluorophenyl)acetate, iPr2NEt, THF, 0°C, then KOtBu, dioxane, 110°C, 23-35% over two steps; c) LiOH.H2O, THF, MeOH, 76-97%; d) 2-aminothiazole, iPr2NEt, HATU, DMF, 55°C, 38-73%; e) aryl amine pinacol boronate, Pd(dppf)Cl2, Na2CO3, 4:1 dioxane:water, 105°C, 49-59%.