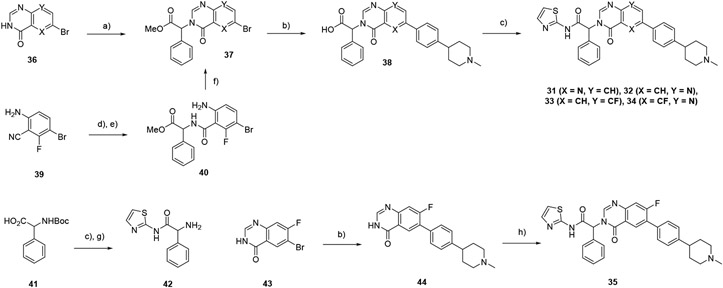
Scheme 3 –
synthesis of compounds 31-35. Reagents and conditions: a) methyl 2-bromo-2-phenylacetate, Cs2CO3, DMF, 50°C, 24-60%; b) 1-methyl-4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl) piperidine, Pd(dppf)Cl2. Na2CO3, 4:1 dioxan:water, 100°C, 11-59%; c) 2-aminothiazole, iPr2NEt, HATU, DMF, 60°C, 9-48%; d) LiOH, water, 100°C, 88%; e) methyl 2-amino-2-phenylacetate, iPr2NEt, HATU, DMF, 72%; f) (EtO)3CH, reflux, 79%; g) TFA, DCM; h) 42, HATU, DBU, CH3CN, 7%.