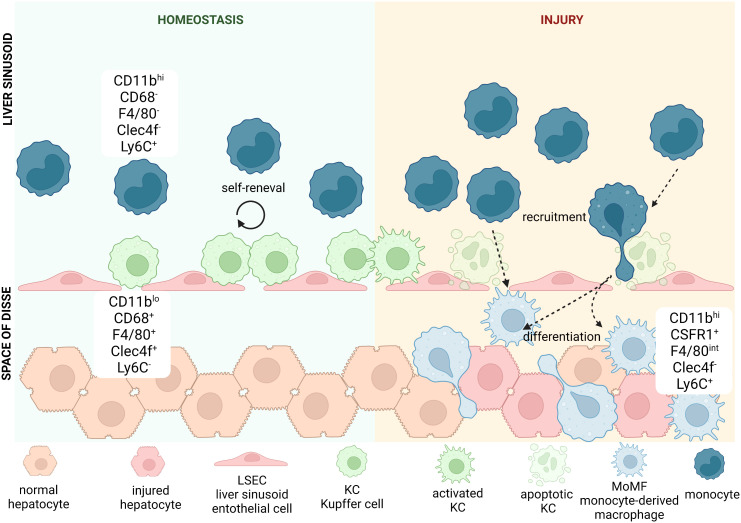
Figure 2.
Heterogeneity of hepatic macrophages. In mice, liver-resident macrophages known as Kupffer cells (KCs) are classically defined by positive expression of CD11b, CD68, F4/80, Clec4f and negativity for Ly6C. They are located in liver sinusoids, where they adhere to liver sinusoid endothelial cells (LSECs). KCs thanks to their particular location remain in close contact with blood stream, which allows them to detect a variety of antigens. During homeostasis the pool of KCs is being replenished by cell renewal. During acute or chronic liver injury KCs get activated and secrete cytokines and chemokines that can recruit other immune cells from the circulating blood. Some of the secreted cytokines or chemokines thanks to fenestrae present between LSECs can reach liver parenchyma and directly affect hepatocytes and other immune cells located there. Haptic injury and/or chronic inflammation increases apoptotic rate of KCs and when self-renewal does not suffice to maintain their population, we can observe increased recruitment of monocytes, characterized by positivity for CD11b and Ly6C, with concomitant negativity to CD68, F4/80 and Clec4f. Once they enter parenchyma through endothelial fenestration, they differentiate into KC-like cells called monocyte-derived macrophages (MoMFs), that resemble the phenotype and function of KCs. These highly pro-inflammatory cells can be recognized by positivity for some markers typical for both KCs and monocytes: CD11b, F4/80 and Ly6C, while they remain negative for Clec4f. They also repopulate hepatic macrophages niche after increased death of KCs due to injury.