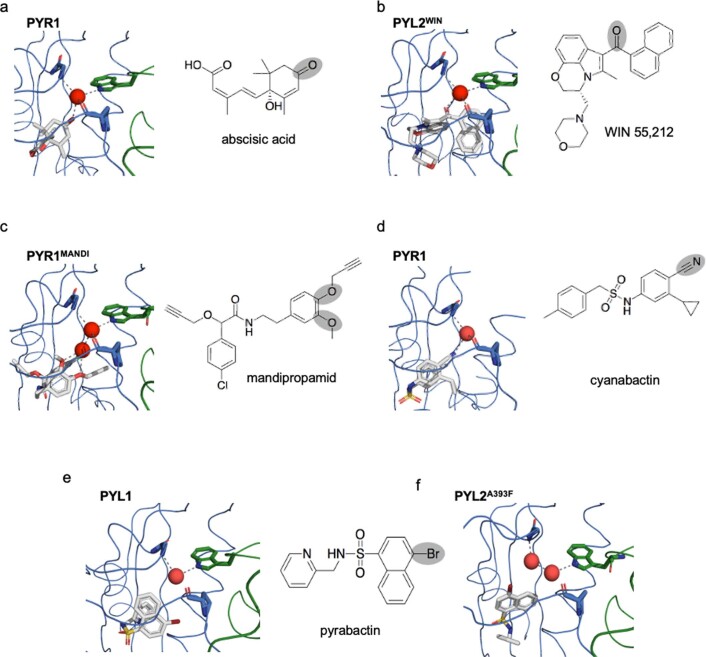
Extended Data Fig. 3. Diverse H-bond acceptors can stabilize activated PYR/PYL receptors.
Diverse H-bond acceptors can stabilize activated PYR/PYL receptors. Structural comparisons of diverse receptor-ligand complexes with the receptor shown in blue and the complexed PP2C (HAB1) is shown in green. (a) In wild-type complexes, the PYR1/ABA/HAB1 complex is stabilized by a hydrogen-bond network coordinated by a central water molecule conserved across ligand-activated receptor X-ray structures (red). The conserved water stabilizes the ternary complex by making contacts to ABA’s cyclohexenone oxygen and PYR1’s P88 main-chain carbonyl oxygen, which serve as H-bond acceptors, and HAB1’s TRP385 indole HN and PYR1’s ARG116 main-chain NH, which serve as H-donors (PDB 3QN1). (b) In the engineered PYL2WIN receptor, WIN 55,212’s naphthoylindole carbonyl oxygen interacts with the conserved water molecule to engage the TRP-lock, mimicking ABA’s carbonyl oxygen. (c) In the engineered PYR1MANDI receptor, mandipropamid’s aryl-alkyl ether oxygens stabilizes the PYR1MANDI/mandipropamid/HAB1 complex via two bound waters that interact with ether oxygens (PDB 4WVO). (d) The rationally designed agonist cyanabactin stabilizes activated PYR1 via an arylnitrile H-bond acceptor that interacts with the conserved water (PDB 5UR6). (e, f) The agonist pyrabactin (with a weak C-Br H-bond acceptor) does not make appreciable contacts with bound water either in homologous PYL1 receptor nor in the engineered PYL2A393F. (e shows PDB ID 3NMT, native PYL1, PDB ID 3NMN is shown in f).