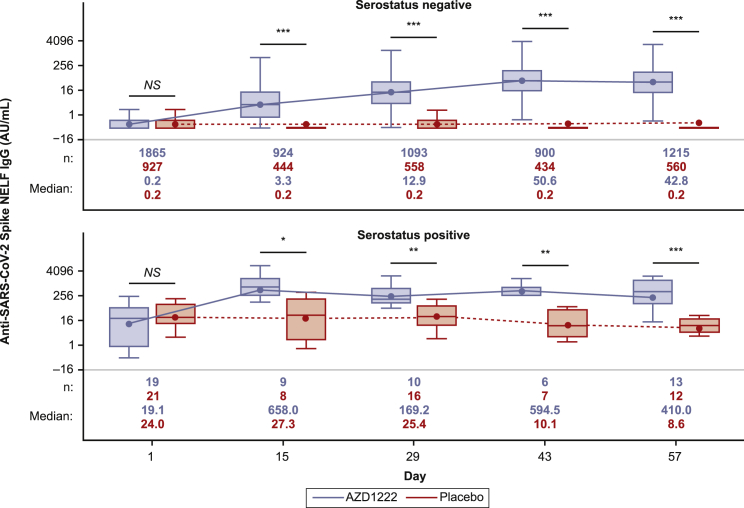
Figure 1.
Quantification of anti-SARS-CoV-2 spike immunoglobulin G (IgG) in nasal epithelial lining fluid (NELF) from immunogenicity substudy participants following AZD1222 vaccination or placebo, by baseline serostatus
Boxplots illustrate anti-SARS-CoV-2 spike IgG titers observed in NELF following AZD1222 vaccination or placebo according to participant baseline serostatus. Results are presented according to baseline SARS-CoV-2 serostatus as determined by the presence of SARS-CoV-2 nucleocapsid antibodies. The x axis denotes days since the first AZD1222 or placebo dose. Day 1 and day 29 samples were obtained prior to administration of AZD1222 or placebo. The box denotes interquartile range (IQR), the horizontal line in the box denotes median, and the marker in the box is the geometric mean titer (GMT). Any points more than 1.5 × IQR from the box were considered outliers and are not displayed. The whiskers that extend from the box indicate the minimum and maximum after removing the outliers. Boxplots were created using the log-normal distribution. To provide comprehensive information about the durability of immunogenicity after vaccination, data were censored in AZD1222 study participants at the time of non-study COVID-19 vaccination and for placebo participants at the earlier of the time of non-study COVID-19 vaccination or unblinding, whichever occurred first. Statistical evidence between groups was determined by post hoc two-tailed Mann-Whitney tests. Not significant (NS), p > 0.05; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001. AU/mL, arbitrary units per milliliter.