Abstract
The novel coronavirus (SARS-CoV-2) is the third coronavirus this century to threaten human health, killing more than two million people globally. Like previous coronaviruses, SARS-CoV-2 is suspected to have wildlife origins and was possibly transmitted to humans via wet markets selling bushmeat (aka harvested wild meat). Thus, an interdisciplinary framework is vital to address the nexus between bushmeat, wet markets, and disease. We reviewed the contemporary scientific literature to: (1) assess disease surveillance efforts within the bushmeat trade and wet markets globally by compiling zoonotic health risks based on primarily serological examinations; and (2) gauge perceptions of health risks associated with bushmeat and wet markets. Of the 58 species of bushmeat investigated across 15 countries in the 52 articles that we analyzed,one or more pathogens (totaling 60 genera of pathogens) were reported in 48 species, while no zoonotic pathogens were reported in 10 species based on serology. Burden of disease data was nearly absent from the articles resulting from our Scopus search, and therefore was not included in our analyses. We also found that perceived health risks associated with bushmeat was low, though we could not perform statistical analyses due to the lack of quantitative perception-based studies. After screening the literature, our results showed that the global distribution of reported bushmeat studies were biased towards Africa, revealing data deficiencies across Asia and South America despite the prevalence of the bushmeat trade across the Global South. Studies targeting implications of the bushmeat trade on human health can help address these data deficiencies across Asia and South America. We further illustrate the need to address the nexus between bushmeat, wet markets, and disease to help prevent future outbreaks of zoonotic diseases under the previously proposed “One Health Framework”, which integrates human, animal, and environmental health. By tackling these three pillars, we discuss the current policy gaps and recommend suitable measures to prevent future disease outbreaks.
Keywords: Wild meat, Global South, Nexus, Healthcare, One health approach, Zoonosis
1. Introduction
The novel coronavirus (SARS-CoV-2) outbreak is possibly the greatest humanitarian crisis since the second World War (Anastassopoulou et al., 2020; Arshad Ali et al., 2020). Three months after its initial reporting in Wuhan, China, the World Health Organization declared the 2019 coronavirus disease a pandemic on March 11, 2020 (Cucinotta and Vanelli, 2020). As of April 10, 2021, more than 135 million people across 192 countries have been infected, and global deaths have surpassed 2.9 million (Dong et al., 2020). For the third time this century, a coronavirus poses a grave threat to human health (Memish et al., 2020; Paden et al., 2018; Perlman, 2020).
The World Health Organization (WHO) defines Zoonosis as any disease or infection that is naturally transmissible from non-human animals to humans, which requires a natural reservoir (WHO, 2020). For example, dromedary camels are suspected to be the animal hosts responsible for the spillover of the 2012 Middle East Respiratory Syndrome Coronavirus (MERS-CoV) (Mohd et al., 2016). However, origins and reservoirs of zoonotic diseases are often difficult to identify (Haydon et al., 2002; Viana et al., 2014). Thus, despite global efforts by international organizations such as the WHO, the origin of the coronavirus that caused the outbreak of severe acute respiratory syndrome in 2002–2004 (SARS) and the novel coronavirus (COVID-19) remain unresolved (Wacharapluesadee et al., 2021). However, there is some evidence suggesting that previous coronaviruses were transmitted to humans from intermediate animal hosts, and thus are suspected cases of zoonosis (Cui et al., 2019; Han et al., 2016; Li, 2013). In 2002–2004, the coronavirus that caused the outbreak of severe acute respiratory syndrome (SARS-CoV-1) likely originated in horseshoe bats (family Rhinolophidae) (He et al., 2014; Hu et al., 2017; Wendong Li et al., 2005); and was likely transmitted to humans from raccoon dogs (Nyctereutes procyonoides) found in live-animal markets (Guan et al., 2003; Wenhui Li et al., 2006; Song et al., 2005). Although the origin has yet to be described in detail, some scientists, including the recent report from the WHO, theorize that the novel coronavirus likely has wildlife origins and spread to humans via wild meat sold at a wet market in Wuhan, China (Andersen et al., 2020; Lu et al., 2020; Maxmen, 2021; Shereen et al., 2020; Tang et al., 2020; Wu et al., 2020). Therefore, the connection between wild meat, wet markets, and zoonotic diseases should be investigated.
In light of the recent COVID-19 pandemic, some have called for bans on wildlife trade and the closure of wet markets altogether. However, others caution that blanket bans on wildlife trade may not necessarily improve pandemic preparedness, while undermining its importance in providing food and financial security for certain communities (Eskew and Carlson, 2020; Roe et al., 2020). Following the outbreak of the Ebola virus disease in West Africa 2013–2016, studies revealed unintended consequences of a wildlife trade ban (Ayegbusi et al., 2016; Bonwitt et al., 2018). Without producing the desired degree of public health awareness, the ban drove illicit activity underground, which not only thwarted surveillance for future disease control efforts, but also weakened community stakeholders' trust in authority and worsened food accessibility for already food-insecure populations (Bonwitt et al., 2018).
Bushmeat (aka wild meat) refers to any non-domesticated animal harvested for food (Nasi et al., 2008) and provides food and financial security to millions of people globally (Nielsen et al., 2018). The national value of the bushmeat trade is estimated to be hundreds of millions USD/year for certain African countries (Bowen-Jones et al., 2003; Davies, 2002; Lescuyer and Nasi, 2016). Bushmeat consumption acts as a significant driver behind the disproportionate loss of wildlife, particularly large mammals and herbivores (Ripple et al., 2016). Estimates suggest that about 273 tonnes of bushmeat are transported from Africa to Europe annually (Chaber et al., 2010) and that globally more than 5 million tonnes of bushmeat is consumed every year (Kanagavel et al., 2016). Animal-source foods are a major source of high quality protein and bioavailable nutrients, especially in the poorer regions of the world (Adesogan et al., 2020; Neumann et al., 2003). On the other hand, livestock, such as intensively-raised commercial meat chickens, provide more energy from fat than protein (Wang et al., 2010), further demonstrating the higher nutritional value of bushmeat (Sarti et al., 2015). Factors that trigger bushmeat consumption include, among others, population growth, poverty, limited market access, war and conflict, unequal wealth distribution, occupation as well as preference of wild meat over farm-grown meat (Brashares et al., 2011; Friant et al., 2020; Golden et al., 2014; Kanagavel et al., 2016; Lindsey et al., 2013; Rogan et al., 2018; Schulte-Herbrüggen et al., 2013).
Conversely, demand for bushmeat consumption in urban areas are often driven by culture and consumer preferences (Chausson et al., 2019; McNamara et al., 2019; van Vliet and Mbazza, 2011). In recent decades, the spike in production, trade, and consumption of meat has tied Asian countries to each other and global markets through meat commodities, leading to the emergence of a meat complex in Asia (Jakobsen and Hansen, 2020; Nam et al., 2010; Sans and Combris, 2015). This Asian meat complex has the potential to exacerbate the health risks associated with meat consumption. For bushmeat consumption, long-term health risks associated with bushmeat consumption include exposure to hazardous levels of heavy metals, such as lead. (Ahmadi et al., 2018; Cang et al., 2004; Gbogbo et al., 2020; Pain et al., 2010). Short-term risks associated with bushmeat consumption may include exposure to zoonotic diseases, which can be fatal (Jones et al., 2008; Karesh and Noble, 2009; Kurpiers et al., 2015). These same risks also occur from animal-source foods in domestic animals, especially those produced intensively (WHO, 2020). The domestic meat market is roughly 60 times larger than the bushmeat market. However, despite the smaller proportionate risks associated with bush meat consumption, there is evidence connecting the bushmeat trade in the spread of infectious diseases including Ebola (Leroy et al., 2004, 2005; Rewar and Mirdha, 2014) and HIV (Aghokeng et al., 2010; Chitnis et al., 2000; Faria et al., 2014; Peeters et al., 2002; Sharp and Hahn, 2011). Despite associated health and injury risks, bushmeat consumption is widespread across various socio-economic communities. Bushmeat is often sold at wet markets across the Global South (Nielsen et al., 2018), which pose additional health risks to humans.
Wet markets and wildlife markets are often conflated. However, not all wet markets sell bushmeat. Therefore, in this paper, we focus on wet markets that include live or slaughtered bushmeat. Generally, wet markets feature densely-packed, open-air vendors that sell produce and various species of bushmeat. Thus, wet markets, like factory-farmed meat, can amplify the risks associated with the bushmeat trade by facilitating cross-species transmissions of zoonotic diseases (Karesh et al., 2005; Parrish et al., 2008). Thus, wet markets have been a major concern of disease experts across the globe (Cui et al., 2019; Webster, 2004). Notably, humans with ties to certain wet markets in Asia have been infected with coronaviruses with suspected animal origins, including the outbreak of the Middle East respiratory syndrome (MERS) in live animal markets (Khudhair et al., 2019; Li et al., 2017; Yusof et al., 2017), SARS (Guan et al., 2003; Li et al., 2006; Park et al., 2020; Song et al., 2005), and COVID-19 in wet markets (Chen et al., 2020; Li et al., 2020).
In developing countries, wet markets/slaughterhouses are a significant source of wastewater or effluents (blood flow, hair, gut content, urine and contaminated water) due to meet processing, burning and boiling of bones, hooves, fat, meat, etc. (Seiyaboh and Izah, 2017; Singh et al., 2014). Based on the water quality index, it is reported that wastewater from wet market falls under the lowest category (i.e., severely polluted), making the receiving water bodies highly vulnerable to organic and inorganic pollutants; and hence calls for sustainable management and treatment of the effluents before discharging into the environment (Amneera et al., 2013). Poor management and treatment of this effluent poses different threats to both environment and human health (Singh et al., 2014). These threats are exacerbated in water-scarce places where humans and wildlife are forced into close proximity for available resources, a contributing risk factor for transmission of water-associated diseases (e.g. leptospirosis) (Group, 2011). Despite known health risks and alternative sources of food, wet markets have persisted in both developed and developing parts of the globe. In developing economies, the lack of reliable cold chains could contribute to the continued presence of wet markets (Joshi et al., 2009). Furthermore, culture and consumer preferences for fresh produce has been cited for the persistence of wet markets globally (Huang et al., 2015; Zhong et al., 2020).
One Health is an interdisciplinary framework that addresses human health, animal health, and environmental health. Recently, this definition has been expanded to include plants and soil ecosystems, linking the One Health approach to food systems (Durso and Cook, 2019). Thus, the One Health approach provides an avenue to combat emerging infectious diseases, especially zoonotic diseases (Atlas, 2013; Mazet et al., 2009). For example, the Ebola crisis could have been better handled with a One Health approach by proactively monitoring infection and transmission, educating stakeholders about zoonotic disease outbreak and management plans, and enhancing food security to reduce dependence on bushmeat and minimize exposure to zoonoses (Mwangi et al., 2016). Applying the One Health approach can guide research to help prevent the emergence of zoonotic diseases by acknowledging the nexus between bushmeat, wet markets, and disease. This nexus approach provides a framework for transformative change by integrating efforts between health and science experts, government officials, and local stakeholders (Eskew and Carlson, 2020; Roe et al., 2020; World Health Organization, 2012). To our knowledge, however, this nexus has not yet been described in detail. Thus, the nexus between bushmeat, wet markets, and disease must be examined to bolster disease control efforts globally and better prepare for future pandemics.
In this study, we reviewed the literature to explore the nexus between bushmeat, wet markets, and disease. Our aim was to evaluate disease monitoring and surveillance efforts within the bushmeat trade by compiling established zoonotic health risks based on serology; and to characterize perceptions of the health risks associated with bushmeat and wet markets to better inform education campaigns and public policy. Based on this review, we provide recommendations to prevent future pandemics by addressing and controlling the nexus between bushmeat, wet markets, and disease control.
2. Methods
2.1. Literature search
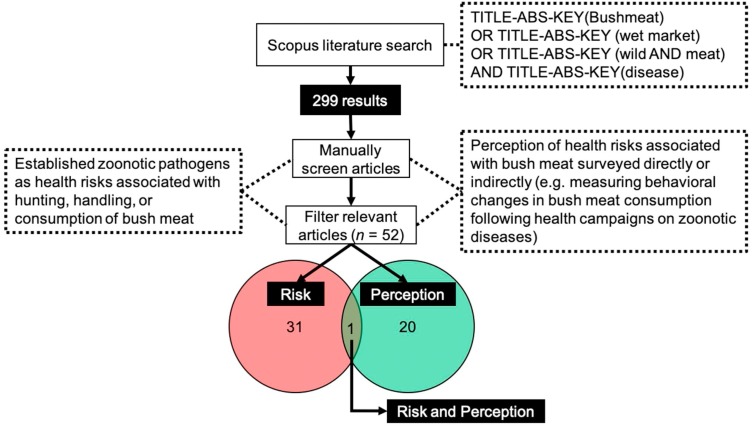
To explore the nexus between bushmeat, wet markets, and disease, we searched the Scopus literature database (https://www.scopus.com/) on November 9, 2020 using the Boolean search string: TITLE-ABS-KEY (Bushmeat) AND TITLE-ABS-KEY (disease) OR TITLE-ABS-KEY (wet market) OR TITLE-ABS-KEY (wild AND meat). The search was not restricted by publication date nor language. However, only articles that provided full text in English were considered for analyses. The search yielded 299 articles (see Appendix 1 for a full list of articles). Eight articles that did not provide full text in English were omitted. Thereafter, we manually screened the resulting article abstracts to include only primary literature that explicitly established zoonotic pathogens (hereafter risk assessment studies) associated with hunting, handling, or consumption of non-domesticated animals, which the author(s) referred to as 'bushmeat' or matched the definition of the term described in the Convention on Biological Diversity (CBD) Technical Series No. 33 (Nasi et al., 2008). Health risks were determined generally by serological evidence. Our Scopus search yielded inadequate scientific disclosures that calculated actual burden of zoonoses, and thus we did not consider disease burden in determining risk of zoonosis. We also included studies that surveyed the perception of health risks associated with bushmeat (hereafter perception-based studies) directly by questioning respondents about their perceived health risks or indirectly, for example, by measuring behavioral changes in bushmeat consumption following health campaigns on zoonotic diseases. We retrieved the full-text of the articles meeting these criteria and manually screened out the articles that did not provide evidence on the same criteria in full-text. After screening abstracts and full-texts, 247 articles were excluded that did not meet the criteria specified above, leaving 52 relevant articles: 32 risk assessment and 21 perception-based studies. One article met the criteria specified for both risk assessment and perception-based studies (Fig. 1 ).
Fig. 1.
Flowchart of methods and Scopus literature screening process.
2.2. Meta-analysis
We extracted the following information from the relevant risk assessment studies that were identified: (1) type and species of bushmeat investigated, (2) location from which the samples were collected, and (3) type and genus of zoonotic pathogen associated with the bushmeat in question. We categorized bushmeat according to broad taxonomic groups. Pathogen type was categorized as virus, bacteria, helminth, or protozoa. For meta-analysis of the extracted information from the relevant risk studies, we counted each species of bushmeat collected from each country in each article as a case. The results presented in the following section derive from cross-tabulation of the attributes of all cases.
For the relevant perception-based studies, we extracted the following information: (1) location from which respondents were surveyed, (2) population type of respondents, (3) type of bushmeat handler surveyed, and (4) perceived health risks associated with the bushmeat trade. We used descriptions of study sites to determine the population type of respondents as either urban or rural. We categorized bushmeat handler as hunter, trader, or other (non-hunter/non-trader). We extracted author's description (both quantitative and qualitative) to categorize respondents' perceived health risks as either high or low. For example, in addition to noting that few participants were aware of transmission of zoonotic infections via bushmeat, Ozioko et al. (2018) reported that 91.2 % and 76.2 % of hunters and traders valued bushmeat more than their health, respectively. For this study, we categorized the perceived health risks as low. On the other hand, we categorized the perceived health risks as high for the study by Gbogbo and Kyei (2017), which reported that 68 % of respondents believed that the consumption of bushmeat can result in zoonotic disease infection. For meta-analysis of information from perception studies, we counted each population type from each country in each article as a case. The results presented in the following section derive from cross-tabulation of the attributes of all cases.
2.3. Data analysis
The data on species of bushmeat were subject to phylogenetic analysis. All figures were constructed in R (v.3.6.2) (R Core Team, 2014). We used the package ‘rotl' (Michonneau et al., 2016) to match taxonomic names to the Open Tree of Life (Hinchliff et al., 2015). The unique taxonomic names from the Open Tree of Life matched the 59 species that we compiled. Using the ott identification tags of these species as search properties, we identified studies that included these unique taxonomic tags and found 9 studies that contained relevant phylogenetic trees. We used existing phylogenies from these studies to construct a phylogenetic tree for the species of bushmeat reported in the relevant risk studies. We used the study (Hedges et al., 2015) that contained a rooted phylogeny with branch lengths and kept tips corresponding to the 59 species. The resulting phylogeny contained 58 tips. The blond capuchin (Sapajus flavius) was the only species that could not be included in our reconstructed phylogenetic tree. The number of cases was mapped onto the phylogeny as a continuous variable. We mapped the global distributions of risk assessment and perception-based studies using the package ‘rworldmap’ (South, 2011).
3. Results
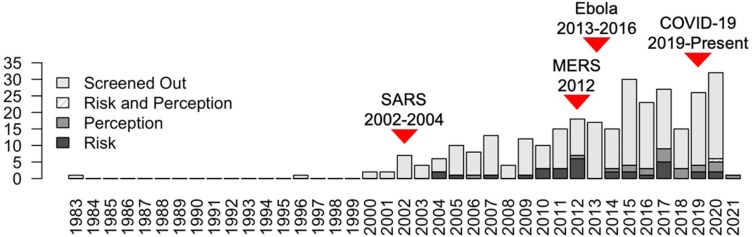
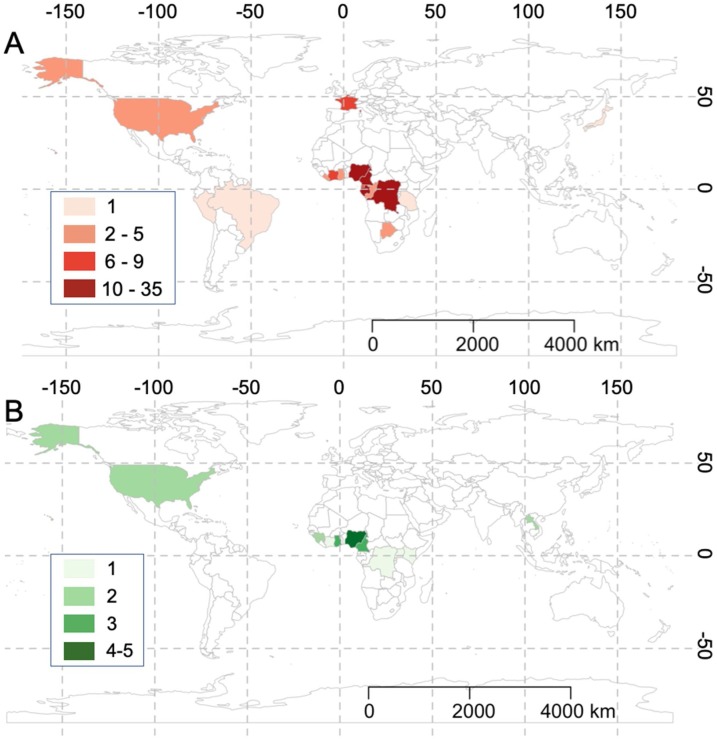
From all 299 Scopus search results, we observed topical spikes in published papers following the SARS epidemic (2002–2004) and MERS epidemic (2009), as well as the African Ebola epidemic (2013–2016) ( Fig. 2 ). After screening irrelevant studies, our analyses included 52 articles, with 88 % of the total cases (137/156 cases) in Africa; 5% (8/156 cases) in Europe; 4% (7/156 cases) in North America; 2% (3/156 cases) in Asia; and 1% (2/156 cases) in South America.
Fig. 2.
Distribution of Scopus search results by publication date. Topical spikes of publications following the outbreak of severe acute respiratory syndrome (SARS), the Middle Easter respiratory syndrome (MERS), and the Ebola virus disease illustrate that science retroactively follows disease outbreaks.
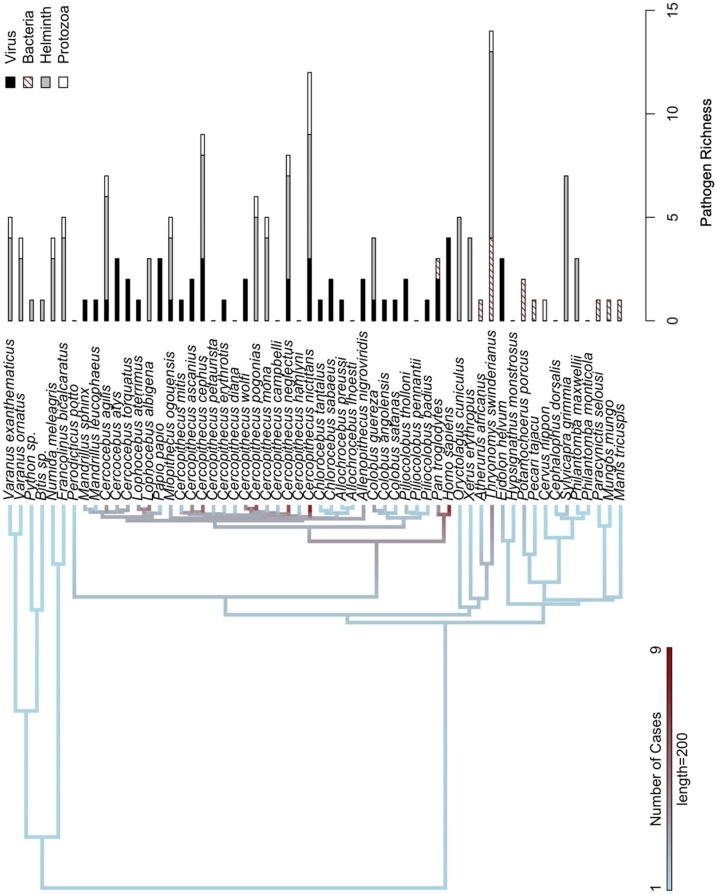
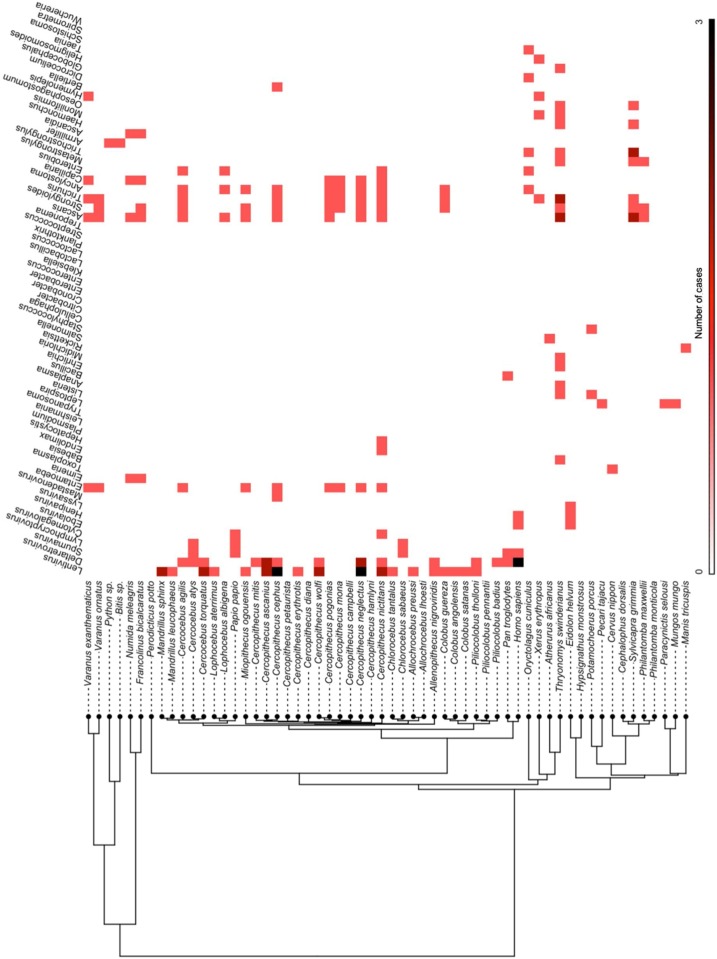
For the 32 risk assessment studies, 58 species of bushmeat (and humans [Homo sapiens]) were studied in 15 countries (Fig. 3 a), totaling 133 cases (see Appendix 2 for a list of the 133 risk assessment cases and the type of pathogenic risk(s) established in each case). Several species were reported in more than one article, and one study was conducted in two countries. Mammals were most frequently reported (95 %; 126/133 cases), followed by reptiles (4%; 5/133 cases) and birds (1%; 2/133 cases). Greater white-nosed monkeys (Cercopithecus nictitans) and humans were cited the most times with 9 and 8 cases, respectively (Fig. 4 and see Appendix 3 for a list of the number of cases and type of pathogen for each species of bushmeat). Based on serological evidence, sixty pathogens were established as health risks in 58 bushmeat species, with several species of bushmeat harboring one or more pathogens; furthermore, ten bushmeat species harbored no zoonotic pathogens. Most of the zoonotic pathogens established as health risks were helminth (37 %; 22/60) and bacteria (33 %; 20/60), followed by viruses and protozoa (15 %; 9/60 each). The predominate zoonotic pathogens identified were retroviruses: Lentivirus (immunodeficiency viruses) and Deltaretrovirus (T-cell lymphotrophic viruses), which were identified in 18 and 14 species of bushmeat, respectively. Other predominate zoonotic pathogens identified were parasitic roundworms Ascaris, Strongyloides, and Trichuris, which were identified in 14, 13, and 12 species of bushmeat, respectively. The predominate protozoa and bacteria identified were Entamoeba and Leptospira, which were identified in 9 and 3 species of bushmeat, respectively (Fig. 5 and see Appendix 4 for a full matrix of bushmeat-zoonotic pathogen infections). Four viruses were identified in humans. Regarding pathogen richness in each species of bushmeat, health risks were highest for greater cane rats and greater white-nosed monkeys, harboring 14 genera of pathogens (1 protozoa, 4 bacteria, and 9 helminth) and 13 genera of pathogens (3 viruses, 4 protozoa, and 6 helminth), respectively.
Fig. 3.
Global distribution of risk assessment (top) and perception-based (bottom) studies on bush meat, represented by the number of cases in each country identified from the literature. Global distributions were depicted using the package ‘rworldmap’ (South, 2011).
Fig. 4.
Total number of cases identified for each species of bush meat mapped onto the phylogeny as a continuous variable. The length of the bars indicates the genus richness of zoonotic pathogens identified for each species of bush meat. Phylogenetic scale bar indicates 200 million years.
Fig. 5.
Heat map of the number of cases for each bush meat-pathogen infection. As one or more pathogens was established in some species of bush meat, the total of number of cases reported for risk assessment studies do not agree with the total number of bush meat-pathogen infection cases here.
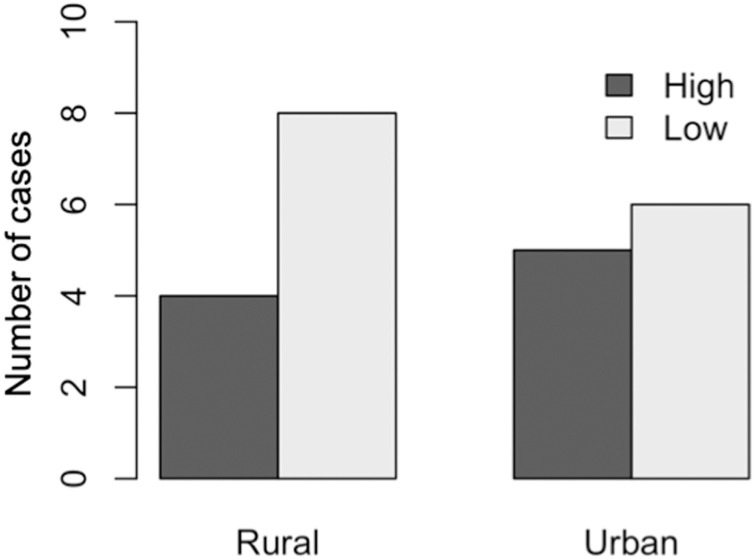
For the 21 perception-based studies, perceived health risks associated with the bushmeat trade was documented in 11 countries (Fig. 3b), totaling 23 cases (see Appendix 5 for a list of the 23 perception cases and the perceived health risk described in each case). Two studies were conducted in both rural and urban population types. Altogether, 12 and 11 cases were reported in rural (52 %) and urban (48 %) population types, respectively. Our analyses found that nearly half (48 %; 11/23 cases) of the perception-based studies were conducted in the context of the Ebola virus disease outbreak. In both urban and rural populations, perceived health risks associated with the bushmeat trade was generally low (Fig. 6 ).
Fig. 6.
Perceived health risks associated with the bush meat trade.
4. Discussion
4.1. Literature compilation
There was a clear dominance of publications following major health crises, such as SARS, MERS, and Ebola, demonstrating that scientific investigations on bushmeat and wet markets retroactively follow disease outbreaks. Furthermore, after screening irrelevant articles, there was geographical bias in the remaining studies, with a majority of studies conducted in Africa. Our analysis clearly revealed studies lacking in Asia and South America, despite the prevalence of the bushmeat trade across the Global South. Altogether, these factors contribute to the speculative narrative surrounding disease outbreaks originating in these regions of the world and expose our knowledge gaps that may thwart disease control efforts.
4.2. Zoonotic pathogens
Although disease burden was not calculated, our findings demonstrate that zoonotic viruses may have the potential to pose significant health risks to humans. Based on serological investigations, we found that bushmeat handlers were infected with four zoonotic viruses: Deltaretrovirus, Spumavirus (foamy viruses), Ebolavirus, and Henipavirus (Nipah virus). Our results showed that these virus were found in various bushmeat species, such as fruit bats (Eidolon helvum) and nonhuman primates, which can thus pose greater risk of spillover events to humans. Accordingly, consumers of these species, which can be vectors of these viruses among other zoonotic pathogens, may be at greater risk of infection. In our study, we found that fruit bats hosted Ebolavirus, Lyssavirus (rabies virus), and Henipavirus. The risks of consuming nonhuman primates are multifold. Infectious viruses are abundant in nonhuman primates (Devaux et al., 2019). Accordingly, the total number of cases for nonhuman primates was high (65 %; 86/133 cases). We found that nonhuman primates hosted Lentivirus, Deltaretrovirus, Spumavirus, Cytomegalovirus, Lymphocryptovirus, and Mastadenovirus. Nonhuman primates may also act as intermediate host species and may transmit other zoonotic diseases to humans (Devaux et al., 2019; Han et al., 2015; Weingartl et al., 2012). By contrast, we found that certain species of bushmeat hosted no zoonotic pathogens based on the serological evidence, which may pose less health risks to humans. These lower-risk species can help provide options for safer consumption of bushmeat. Altogether, our results demonstrate how bushmeat-pathogen matrices can inform bushmeat regulation policies and visualize disease surveillance efforts.
4.3. Perceptions
Despite multiple disease outbreaks globally and well-documented zoonotic diseases associated with bushmeat, we found that perceived health risks are typically low among bushmeat handlers. We found that, in some cases, simply awareness of zoonoses among respondents was low (Ozioko et al., 2018; Philavong et al., 2020; Pruvot et al., 2019). We also found that there was a critical knowledge gap between awareness of disease and mode of transmission to humans, which can serve an important role in shaping perceived risks associated with bushmeat (Ayegbusi et al., 2016; Bair-Brake et al., 2014; Duonamou et al., 2020; Lucas et al., 2020; Mwangi et al., 2016; Saylors et al., 2021; Subramanian, 2012). For example, one perception-based study found a high level of awareness about the Ebola virus disease outbreak among bushmeat handlers, however, most of the handlers did not believe that wild animals are carriers of Ebola virus disease, citing supernatural and conspiracy theories surrounding its transmission to humans (Ayegbusi et al., 2016). Regarding perceived health risks, the skepticism of respondents from other studies stem from cultural ties to bushmeat practices (Ayegbusi et al., 2016; Bonwitt et al., 2018; Saylors et al., 2021). These views were conflated with trust issues in local authorities, which ultimately frustrated public health campaigns (Ayegbusi et al., 2016; Bonwitt et al., 2018; Saylors et al., 2021). Our analysis on perception-based studies found a deficiency of quantitative reporting, which prevented statistical modeling in our meta-analyses. We highlight the need for standardizing perception-based studies that are grounded in quantitative analyses. Quantitative data is necessary to inform educational campaigns aimed at raising awareness and conveying zoonotic risks associated with bushmeat.
4.4. Importance of nexus approach
An interdisciplinary framework is vital to address the nexus between bushmeat, wet markets, and disease. There is extensive research on each of these three topics in isolation, however, few investigations have explored these topics together. Additionally, oversight of these topics is often handled separately by different governing bodies. For example, budgets and policies on bushmeat likely fall under the responsibility of wildlife departments; wet markets likely by commerce departments; and health departments likely handle diseases. However, these isolated efforts fail to acknowledge the synergistic risks induced at the interface of these three issues, as exemplified by the current COVID-19 pandemic. Thus, efforts that extend across the borders of traditional governing bodies is paramount to properly address this nexus. Effective campaigns require coordinated efforts between health and science experts, government officials, and local stakeholders (Roe et al., 2020; Van Vliet, 2011), which is especially important for those communities whose livelihoods depend on the bushmeat trade (Cooney et al., 2018; Friant et al., 2020). The One Health approach by World Health Organization, which addresses human, animal, and environmental health, provides a framework to improve pandemic preparedness across the globe. The One Health framework recognizes that zoonotic diseases, environmental pressures, animal and human health are linked interdependently. As such, a recent study implicated biodiversity loss with increased risk of human exposure to both new and established pathogens (Keesing and Ostfeld, 2021), further demonstrating the need to assess the impacts anthropogenic land-use change on disease spillover (Plowright et al., 2021). In Industrial safety engineering, Fire Triangle is a model that illustrates how to extinguish fires by removing one of its three necessary ingredients: oxygen, heat, and fuel. Similarly, we argue that future disease outbreaks can be prevented by tackling what we call the three pillars of pandemics: bushmeat, wet markets, and disease. A majority (72 %) of emerging infectious diseases are zoonotic (Jones et al., 2008). Opportunities for transmission of zoonotic diseases increase with the hunting, handling, and consumption of bushmeat (Karesh and Noble, 2009; Kurpiers et al., 2015). Further closing the gaps between human and wildlife, wet markets facilitate cross-species disease transmission and spillover events to humans (Chen et al., 2020). Thus, these synergistic effects underscore the importance of acknowledging the nexus between bushmeat, wet markets, and disease to help prevent future pandemics.
5. Recommendations
Here we discuss some preventative actions outlining bushmeat consumption, management of wet markets, and controlling disease outbreaks, particularly on the overlapping interfaces. Given the global distribution of bushmeat consumption and wet markets, we provide generic recommendations under the One Health framework that can facilitate transformative change and instigate localized efforts to address context-specific issues.
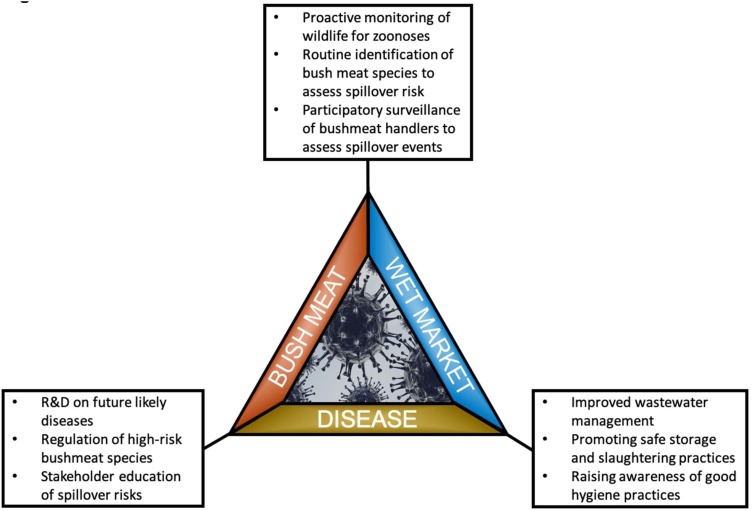
As shown in Fig. 7 , proactive management and regulation of the bushmeat trade can benefit both wildlife and humans. We recommend routine monitoring of wildlife for infectious diseases (Watsa, 2020) in tandem with the Convention on International Trade in Endangered Species and Wild Fauna and Flora (CITES) (D’Cruze and Macdonald, 2016) to better inform bushmeat trade regulations. This may not only reduce spillover events to humans (Halliday et al., 2012; Hattendorf et al., 2017), but also provide information on overall ecosystem health (Leroy et al., 2004; Smith et al., 2009; Thompson et al., 2010). Illegal bushmeat hunting is an existential threat for some species, particularly primates (Benítez-López et al., 2017; Ripple et al., 2016; Rogan et al., 2017). At the national level, regulating the bushmeat trade for higher-risk and endangered species is vital to ensure both animal and human health. Monitoring wildlife for disease may also highlight species suitable for lower-risk consumption of bushmeat, as our results showed that no zoonotic pathogens were associated with other bushmeat species based on serological evidence. We also recommend participatory surveillance of bushmeat sold at wet markets. Species of bushmeat are sometimes misreported in the marketplace (Minhós et al., 2013; Schilling et al., 2020). Even worse, a recent article illustrated the concept of "species deception," in which bushmeat is purposefully misrepresented by sellers and sold as another species of bushmeat (Dell et al., 2020). This can contribute to misinformed knowledge of pathogen spillover risk to humans. Species identification of bushmeat sold in wet markets can also support efforts to better regulate the bushmeat trade. Stakeholder representation, implementing concrete monitoring and evaluation structures, and mutual understandings of disease transmission across disciplines are challenges to implementing a One Health approach (Johnson et al., 2018; Khan et al., 2018). Benefits of participatory monitoring help address these challenges, and include developing research capacity, fostering stakeholder relationships, and earlier detection of emerging infectious diseases (Mooney-Somers and Maher, 2009). Integrating bushmeat policies into CITES enforcement can help consolidate efforts to safeguard both wildlife and human health.
Fig. 7.
A conceptual framework under the ‘One Health’ approach to prevent future disease outbreaks by tackling the three pillars of pandemics: bush meat, wet markets, and disease.
Wet market policies should focus on sanitization and safe meat storage. The temperature storage and packing conditions impact spoilage and the proliferation of subsequent pathogen populations, particularly bacteria (Chaillou et al., 2015; Doulgeraki et al., 2012). These microbial developments generate volatile organic compounds, which may serve as indicators of meat spoilage (Casaburi et al., 2015). Proper wastewater management from wet markets can minimize threats to humans and surrounding ecosystems. We recommend harnessing wastewater as an alternative superior medium for micro algae biomass (Jais et al., 2015; Maizatul et al., 2017). Microalgae biomass produced during the phycoremediation of wastewater from wet markets provides high quality fish food and reduces the dependency on freshwater for production of micro-algal biomass. Management of wastewater from wet markets around the world have proven successful among different advanced wastewater treatment technologies, like Static granular bed reactor with anaerobic reactor (Debik and Coskun, 2009); Up-flow anaerobic sludge blanket reactors (UASB) reactors (Menezes Lima et al., 2020); Dissolved-air floatation system (de Nardi et al., 2008); and Biological wastewater treatment with combination of anaerobic and aerobic system (Aziz et al., 2019). These treatment technologies not only manage effluent from wet markets but also generate biogas energy (Bustillo-Lecompte and Mehrvar, 2015), and thus provide a sustainable solution to wastewater management. Wastewater generation should be minimized by analyzing their quality at critical stages of production process in wet markets using qualitative and quantitative flow charts (Kist et al., 2009). A decade long study on market characteristics revealed poor sanitization conditions and cross-species blood contamination due to inadequate water availability (Saylors et al., 2021). Also, our findings suggest that those who travel to wet markets, including bushmeat vendors, are not aware of these health risks (Ozioko et al., 2018; Philavong et al., 2020; Pruvot et al., 2019; Saylors et al., 2021). Thus, investments in water supply coupled with increased accessibility to washing stations can minimize health risks to both humans and the surrounding ecosystems (Saylors et al., 2021).
Finally, we stress the importance of fostering preventative healthcare infrastructures across national boundaries to improve global pandemic preparedness (Dey et al., 2020; Lal et al., 2020; Yager et al., 2008). The COVID-19 pandemic has showcased the shortfalls of current systems, crippling healthcare infrastructures across the world. Low testing capabilities thwarted efforts to contain the spread of the disease (Babiker et al., 2020), and infected patients were not able to receive proper care due to inadequate hospital capacities (Moghadas et al., 2020; Shoukat et al., 2020). Accordingly, a study found that increasing testing and hospital beds, as well as improving government effectiveness, are associated with lower mortality rates (Liang et al., 2020). Although the timely development of effective vaccines has been possible in the past (Kieny, 2018; Peeling et al., 2019; Roberts, 2019), even in the case of the COVID-19 (Krammer, 2020), funding poses a major barrier for widespread vaccination (Gouglas et al., 2018). Furthermore, socio-economic disparities accentuate the challenges in vaccine development, manufacturing, and delivery at the local and global level (Plotkin et al., 2017). Thus, we recommend proactive research and development to face future likely diseases. Despite having the technological capacity to improve pandemic preparedness, proactive strategies currently do not provide economic stability, failing to prioritize immunologics, such as vaccine or antibody developers (Bloom et al., 2017). This underscores the necessity of routine wildlife surveillance for diseases, which can position stakeholders to better invest in proactive research and development efforts. We also recommend campaigns to increase awareness of the health risks associated with bushmeat, including the transmission of diseases. We found a critical knowledge gap between awareness of disease and spillover risks to humans (Ayegbusi et al., 2016; Bair-Brake et al., 2014; Duonamou et al., 2020; Lucas et al., 2020; Mwangi et al., 2016; Saylors et al., 2021; Subramanian, 2012). This presents an opportunity to not only raise the awareness on health risks associated with bushmeat but more importantly educate bushmeat stakeholders on cross-species transmission of zoonotic diseases. Stakeholders must be more informed decisions on bushmeat practices in order to minimize the risks of future pandemics.
6. Conclusion
Assessing the risks of future disease outbreaks require an interdisciplinary approach, such as the previously described, “One Health Framework”, which integrates human, animal, and environmental health. The One Health Framework provides a lens to address what we call the three pillars of pandemics: bushmeat, wet markets, and disease. Although these topics are extensively studied in their respective disciplines, rarely do studies consider this nexus in preparing for future disease outbreaks. In this directed and systematic review, our findings show that scientific investigations on these topics follow major disease outbreaks, which inhibit investments in future pandemic research. Furthermore, the lack of studies in Asia and South America exposes current knowledge gaps and our susceptibility to disease outbreaks originating in these regions of the world. We also demonstrate how bushmeat-pathogen infection matrices can help regulate the hunting and trade of wildlife in tandem with current CITES policies. Despite evidence of bushmeat species harboring various zoonotic pathogens in scientific disclosures, the low perception of risks among stakeholders of the bushmeat trade highlights the importance of educating stakeholders about transmission risks of zoonoses as a tool to implement a proactive One Health approach. Acknowledging the nexus between bushmeat, wet markets, and disease is pivotal to improve pandemic preparedness.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The article has not been submitted elsewhere.
Acknowledgements
The work was supported by the Strategic Research and Development Area (JPMEERF16S11500) project financed by the Environment Research and Technology Development Fund of the Environmental Restoration and Conservation Agency of Japan (ERCA).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.envsci.2021.05.025.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Adesogan Adegbola T., et al. “Animal source foods: sustainability problem or malnutrition and sustainability solution? Perspective matters.”. Glob. Food Sec. 2020 [Google Scholar]
- Aghokeng Avelin F., et al. Extensive survey on the prevalence and genetic diversity of SIVs in primate bushmeat provides insights into risks for potential new cross-species transmissions.”. Infect. Genet. Evol. 2010 doi: 10.1016/j.meegid.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi Shukrullah, et al. “Hunting, sale, and consumption of bushmeat killed by lead-based ammunition in Benin.”. Int. J. Environ. Res. Public Health. 2018 doi: 10.3390/ijerph15061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amneera W.A., Najib N.W.A.Z., Mohd Yusof S.R., Ragunathan S. “Water quality index of Perlis River, Malaysia.”. Int. J. Civ. Environ. Eng. 2013 [Google Scholar]
- Anastassopoulou Cleo, Russo Lucia, Tsakris Athanasios, Siettos Constantinos. “Data-Based analysis, modelling and forecasting of the COVID-19 outbreak.”. PLoS One. 2020 doi: 10.1371/journal.pone.0230405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen Kristian G., et al. “The proximal origin of SARS-CoV-2.”. Nat. Med. 2020 doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad Ali Shajeea, et al. “The outbreak of coronavirus disease 2019 (COVID-19)—an emerging global health threat.”. J. Infect. Public Health. 2020 doi: 10.1016/j.jiph.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas Ronald M. “One health: its origins and future.”. Curr. Top. Microbiol. Immunol. 2013 doi: 10.1007/82_2012_223. [DOI] [PubMed] [Google Scholar]
- Ayegbusi T., Jegede S.A., Aminu K., Oluwayelu D.O. “Perception and prevention practices against ebola virus disease by Bush meat handlers in Ibadan, Nigeria.”. Afr. J. Biomed. Res. 2016 [Google Scholar]
- Aziz Asad, et al. “Biological wastewater treatment (Anaerobic-Aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater.”. Sci. Total Environ. 2019 doi: 10.1016/j.scitotenv.2019.05.295. [DOI] [PubMed] [Google Scholar]
- Babiker Ahmed, Myers Charlie, Hill Charles, Guarner Jeannette. “SARS-CoV-2 testing: trials and tribulations.”. Am. J. Clin. Pathol. 2020 doi: 10.1093/ajcp/aqaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair-Brake H., et al. “Is that a rodent in your luggage? A mixed method approach to describe bushmeat importation into the United States.”. Zoonoses Public Health. 2014 doi: 10.1111/zph.12050. [DOI] [PubMed] [Google Scholar]
- Benítez-López A., et al. “The impact of hunting on tropical mammal and bird populations.”. Science. 2017 doi: 10.1126/science.aaj1891. [DOI] [PubMed] [Google Scholar]
- Bloom David E., Black Steven, Rappuoli Rino. Emerging infectious diseases: a proactive approach.”. Proc. Natl. Acad. Sci. U. S. A. 2017 doi: 10.1073/pnas.1701410114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonwitt Jesse, et al. “Unintended consequences of the ‘Bushmeat ban’ in West Africa during the 2013–2016 ebola virus disease epidemic.”. Soc. Sci. Med. 2018:200. doi: 10.1016/j.socscimed.2017.12.028. [DOI] [PubMed] [Google Scholar]
- Bowen-Jones E., Brown D., Robinson E.J.Z. 2003. “Economic Commodity or Environmental Crisis? An Interdisciplinary Approach to Analysing the Bushmeat Trade in Central and West Africa.” Area. [Google Scholar]
- Brashares Justin S., et al. “Economic and geographic drivers of wildlife consumption in Rural Africa.”. Proc. Natl. Acad. Sci. U. S. A. 2011 doi: 10.1073/pnas.1011526108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo-Lecompte Ciro Fernando, Mehrvar Mehrab. “Slaughterhouse wastewater characteristics, treatment, and management in the meat processing industry: a review on trends and advances.”. J. Environ. Manage. 2015 doi: 10.1016/j.jenvman.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Cang Long, Jun Wang Yu, Zhou Dong Mei, Dong Yuan Hua. “Heavy metals pollution in poultry and livestock feeds and manures under intensive farming in Jiangsu Province, China.”. J. Environ. Sci. 2004 [PubMed] [Google Scholar]
- Casaburi Annalisa, et al. “Bacterial populations and the volatilome associated to meat spoilage.”. Food Microbiol. 2015 doi: 10.1016/j.fm.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Chaber Anne Lise, et al. “The scale of illegal meat importation from Africa to Europe via Paris.”. Conserv. Lett. 2010 [Google Scholar]
- Chaillou Stéphane, et al. “Origin and ecological selection of core and food-specific bacterial communities associated with meat and seafood spoilage.”. ISME J. 2015 doi: 10.1038/ismej.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausson Alexandre M., et al. “Understanding the sociocultural drivers of urban bushmeat consumption for behavior change interventions in Pointe Noire, Republic of Congo.”. Hum. Ecol. 2019 [Google Scholar]
- Chen Nanshan, et al. “Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study.”. Lancet. 2020;395(10223) doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis Amit, Rawls Diana, Moore Jim. “Origin of HIV type 1 in colonial French equatorial Africa?”. AIDS Res. Hum. Retroviruses. 2000;16(1) doi: 10.1089/088922200309548. [DOI] [PubMed] [Google Scholar]
- Cooney R., Roe D., Dublin H., Booker F. 2018. United Nations Environment Programme Wild Life, Wild Livelihoods: Involving Communities in Sustainable Wildlife Management and Combatting the Illegal Wildlife Trade. [Google Scholar]
- Cucinotta Domenico, Vanelli Maurizio. “WHO declares COVID-19 a pandemic”. Acta Biomedica. 2020 doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Jie, Li Fang, Shi Zheng Li. “Origin and evolution of pathogenic coronaviruses”. Nat. Rev. Microbiol. 2019 doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruze Neil, Macdonald David W. “A review of global trends in CITES live wildlife confiscations”. Nat. Conserv. 2016 [Google Scholar]
- Davies Glyn. “Bushmeat and international development”. Conserv. Biol. 2002;16(3) [Google Scholar]
- de Nardi I.R., Fuzi T.P., Del Nery V. Performance evaluation and operating strategies of dissolved-air flotation system treating poultry slaughterhouse wastewater. Resour. Conserv. Recycl. 2008 [Google Scholar]
- Debik E., Coskun T. “Use of the static granular bed reactor (SGBR) with anaerobic sludge to treat poultry slaughterhouse wastewater and kinetic modeling.”. Bioresour. Technol. 2009 doi: 10.1016/j.biortech.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Dell Bree Anna M., Souza Marcy J., Willcox Adam S. “Attitudes, practices, and zoonoses awareness of community members involved in the bushmeat trade near Murchison Falls National Park, Northern Uganda.”. PLoS One. 2020 doi: 10.1371/journal.pone.0239599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux Christian A., Mediannikov Oleg, Medkour Hacene, Raoult Didier. “Infectious disease risk across the growing human-non human primate interface: a review of the evidence”. Front. Public Health. 2019:7. doi: 10.3389/fpubh.2019.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey Sukhen, Cheng Qiang, Joseph Tan. “All for one and one for all: why a pandemic preparedness league of nations?”. Health Policy Technol. 2020 doi: 10.1016/j.hlpt.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Ensheng, Du Hongru, Gardner Lauren. “An interactive web-based dashboard to track COVID-19 in real time.”. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulgeraki Agapi I., Ercolini Danilo, Villani Francesco, Nychas George John E. “Spoilage microbiota associated to the storage of raw meat in different conditions.”. Int. J. Food Microbiol. 2012 doi: 10.1016/j.ijfoodmicro.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Duonamou Lucie, et al. Consumer perceptions and reported wild and domestic meat and fish consumption behavior during the Ebola epidemic in guinea, West Africa.”. PeerJ. 2020 doi: 10.7717/peerj.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durso Lisa M., Cook Kimberly L. “One health and antibiotic resistance in agroecosystems”. EcoHealth. 2019 doi: 10.1007/s10393-018-1324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskew Evan A., Carlson Colin J. “Overselling wildlife trade bans will not bolster conservation or pandemic preparedness.”. Lancet Planet. Health. 2020;4(6) doi: 10.1016/S2542-5196(20)30123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria Nuno R., et al. “The early spread and epidemic ignition of HIV-1 in human populations.”. Science. 2014 doi: 10.1126/science.1256739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friant Sagan, et al. “Eating bushmeat improves food security in a biodiversity and infectious disease ‘Hotspot.’”. EcoHealth. 2020 doi: 10.1007/s10393-020-01473-0. [DOI] [PubMed] [Google Scholar]
- Gbogbo F., Kyei M.O. “Knowledge, perceptions and attitude of a community living around a colony of straw-coloured fruit bats (Eidolon helvum) in Ghana after ebola virus disease outbreak in West Africa.”. Zoonoses Public Health. 2017 doi: 10.1111/zph.12357. [DOI] [PubMed] [Google Scholar]
- Gbogbo Francis, et al. “Health risk assessment for human exposure to trace metals via bushmeat in Ghana.”. Biol. Trace Elem. Res. 2020;196(2) doi: 10.1007/s12011-019-01953-7. [DOI] [PubMed] [Google Scholar]
- Golden Christopher D., et al. “Economic valuation of subsistence harvest of wildlife in Madagascar.”. Conserv. Biol. 2014 doi: 10.1111/cobi.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouglas Dimitrios, et al. “Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study.”. Lancet Glob. Health. 2018 doi: 10.1016/S2214-109X(18)30346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group W.H.O.Expert. World Health Organization; 2011. “Report of the Second Meeting of the Leptospirosis Burden Epidemiology Reference Group.”. [Google Scholar]
- Guan Y., et al. “Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China.”. Science. 2003 doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Halliday Jo, et al. “Bringing together emerging and endemic zoonoses surveillance: shared challenges and a common solution.”. Philos. Trans. R. Soc. B: Biol. Sci. 2012 doi: 10.1098/rstb.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Hui Ju, et al. “Bats as reservoirs of severe emerging infectious diseases.”. Virus Res. 2015 doi: 10.1016/j.virusres.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Hui Ju, Yu Hao, Xue Jie Yu. “Evidence for zoonotic origins of middle east respiratory syndrome coronavirus.”. J. Gen. Virol. 2016 doi: 10.1099/jgv.0.000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattendorf Jan, Bardosh Kevin Louis, Zinsstag Jakob. One health and its practical implications for surveillance of endemic zoonotic diseases in resource limited settings. Acta Trop. 2017:165. doi: 10.1016/j.actatropica.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Haydon Daniel T., Cleaveland Sarah, Taylor Louise H., Karen Laurenson M. “Identifying reservoirs of infection: a conceptual and practical challenge.”. Emerging Infect. Dis. 2002;8(12):1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., et al. “Identification of diverse alphacoronaviruses and genomic characterization of a novel severe acute respiratory syndrome-like coronavirus from bats in China.”. J. Virol. 2014 doi: 10.1128/JVI.00631-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges S.Blair, et al. “Tree of life reveals clock-like speciation and diversification.”. Mol. Biol. Evol. 2015;32(4) doi: 10.1093/molbev/msv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliff Cody E., et al. “Synthesis of phylogeny and taxonomy into a comprehensive tree of life.”. Proc. Natl. Acad. Sci. U. S. A. 2015;112(41) doi: 10.1073/pnas.1423041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Ben, et al. “Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus.”. PLoS Pathog. 2017 doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Chi Tsun, Tsai Kuen Hung, Chih Chen Yu. “How do wet markets still survive in Taiwan?”. Br. Food J. 2015 [Google Scholar]
- Jais bte, Maisara Noor, Mohamed Radin Maya Saphirabte Radin, Apandi Wan Asma Wan Mohamad, Peralta Hazel Monica Matias. “Removal of nutrients and selected heavy metals in wet market wastewater by using microalgae Scenedesmus Sp.”. Appl. Mech. Mater. 2015 [Google Scholar]
- Jakobsen Jostein, Hansen Arve. Geographies of meatification: an emerging asian meat complex. Globalizations. 2020 [Google Scholar]
- Johnson I., Hansen A., Bi P. The challenges of implementing an integrated one health surveillance system in Australia. Zoonoses Public Health. 2018 doi: 10.1111/zph.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones Kate E., et al. “Global trends in emerging infectious diseases.”. Nature. 2008;451(7181) doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi Rohit, Banwet Devinder Kumar, Shankar Ravi. “Indian cold chain: modeling the inhibitors.”. Br. Food J. 2009 [Google Scholar]
- Kanagavel Arun, Parvathy Sethu, Nameer Paingamadathil Ommer, Raghavan Rajeev. “Conservation implications of wildlife utilization by indigenous communities in the southern western ghats of India.”. J. Asia-Pacific Biodivers. 2016 [Google Scholar]
- Karesh William B., Noble Eric. The bushmeat trade: increased opportunities for transmission of zoonotic disease. Mount Sinai J. Med. 2009 doi: 10.1002/msj.20139. [DOI] [PubMed] [Google Scholar]
- Karesh William B., Cook Robert A., Bennett Elizabeth L., Newcomb James. “Wildlife trade and global disease emergence.”. Emerging Infect. Dis. 2005;11(7) doi: 10.3201/eid1107.050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing Felicia, Ostfeld Richard S. Vol. 118. 2021. pp. 1–8. (Impacts of Biodiversity and Biodiversity Loss on Zoonotic Diseases). (17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Mishal S., et al. “The growth and strategic functioning of one health networks: a systematic analysis.”. Lancet Planet. Health. 2018 doi: 10.1016/S2542-5196(18)30084-6. [DOI] [PubMed] [Google Scholar]
- Khudhair Ahmed, et al. “Risk factors for MERS-CoV seropositivity among animal market and slaughterhouse workers, Abu Dhabi, United Arab Emirates, 2014–2017.”. Emerging Infect. Dis. 2019 doi: 10.3201/eid2505.181728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieny Marie Paule. “Lessons learned from ebola vaccine R&D during a public health emergency.”. Hum. Vaccin. Immunother. 2018 doi: 10.1080/21645515.2018.1442161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kist Lourdes Teresinha, Moutaqi Said El, Machado Ênio Leandro. “Cleaner production in the management of water use at a poultry slaughterhouse of vale do taquari, Brazil: a case study.”. J. Clean. Prod. 2009 [Google Scholar]
- Krammer Florian. “SARS-CoV-2 vaccines in development.”. Nature. 2020 doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- Kurpiers Laura A., Schulte-Herbrüggen Björn, Ejotre Imran, Reeder Dee Ann M. Roblematic Wildlife: A Cross-disciplinary Approach. 2015. Bushmeat and emerging infectious diseases: lessons from Africa. [Google Scholar]
- Lal Arush, et al. Optimizing pandemic preparedness and response through health information systems: lessons learned from ebola to COVID-19. Disaster Med. Public Health Prep. 2020 doi: 10.1017/dmp.2020.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy Eric M., et al. “Multiple ebola virus transmission events and rapid decline of central african wildlife.”. Science. 2004;303(5656) doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- Leroy Eric M., et al. Fruit bats as reservoirs of ebola virus. Nature. 2005 doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- Lescuyer G., Nasi R. Financial and economic values of bushmeat in rural and urban livelihoods in Cameroon: inputs to the development of public policy. Int. For. Rev. 2016 [Google Scholar]
- Li Fang. Receptor recognition and cross-species infections of SARS coronavirus. Antiviral Res. 2013 doi: 10.1016/j.antiviral.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Wendong, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005 doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Li Wenhui, et al. Animal origins of the severe acute respiratory syndrome coronavirus: insight from ACE2-S-Protein interactions. J. Virol. 2006 doi: 10.1128/JVI.80.9.4211-4219.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Yan, et al. Identification of diverse viruses in upper respiratory samples in dromedary camels from United Arab Emirates. PLoS One. 2017 doi: 10.1371/journal.pone.0184718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Qun, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Li Lin, Tseng Ching Hung, Ho Hsiu J., Chun Ying Wu. Covid-19 mortality is negatively associated with test number and government effectiveness. Sci. Rep. 2020 doi: 10.1038/s41598-020-68862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima Menezes, Aline Jessica, Filho Fernando Jorge Correa Magalhães, Constantino Michel, Formagini Edinéia Lazarotto. Techno-economic and performance evaluation of energy production by anaerobic digestion in Brazil: bovine, swine and poultry slaughterhouse effluents. J. Clean. Prod. 2020 [Google Scholar]
- Lindsey Peter Andrew, et al. The bushmeat trade in African Savannas: impacts, drivers, and possible solutions. Biol. Conserv. 2013 [Google Scholar]
- Lu Roujian, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for Virus origins and receptor binding. Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas Ashley, et al. Serology and behavioral perspectives on ebola virus disease among bushmeat vendors in equateur, Democratic Republic of the Congo, after the 2018 outbreak. Open Forum Infect. Dis. 2020 doi: 10.1093/ofid/ofaa295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizatul A.Y., Mohamed Radin Maya Saphira Radin, Al-Gheethi Adel A., Amir Hashim M.K. An overview of the utilisation of microalgae biomass derived from nutrient recycling of wet market wastewater and slaughterhouse wastewater. Int. Aquat. Res. 2017 [Google Scholar]
- Maxmen Amy. WHO report into COVID pandemic origins zeroes in on animal markets, not labs. Nature. 2021 doi: 10.1038/d41586-021-00865-8. http://www.ncbi.nlm.nih.gov/pubmed/33785930 (April 2, 2021) [DOI] [PubMed] [Google Scholar]
- Mazet Jonna A.K., et al. A ‘One health’ approach to address emerging zoonoses: the HALI project in Tanzania. PLoS Med. 2009 doi: 10.1371/journal.pmed.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara James, Fa John E., Ntiamoa-Baidu Yaa. Understanding drivers of urban bushmeat demand in a ghanaian market. Biol. Conserv. 2019 [Google Scholar]
- Memish Ziad A., Perlman Stanley, Van Kerkhove Maria D., Zumla Alimuddin. Middle east respiratory syndrome. Lancet. 2020 doi: 10.1016/S0140-6736(19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michonneau François, Brown Joseph W., Winter David J. Rotl: an r package to interact with the open tree of life data. Methods Ecol. Evol. 2016;7(12) [Google Scholar]
- Minhós T.ânia, et al. DNA identification of primate bushmeat from urban markets in Guinea-Bissau and its implications for conservation. Biol. Conserv. 2013:167. [Google Scholar]
- Moghadas Seyed M., et al. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc. Natl. Acad. Sci. U. S. A. 2020 doi: 10.1073/pnas.2004064117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd Hamzah A., Al-Tawfiq Jaffar A., Memish Ziad A. Middle east respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir Susanna lau. Virol. J. 2016;13(1):1–7. doi: 10.1186/s12985-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney-Somers Julie, Maher Lisa. The indigenous resiliency project: a worked example of community-based participatory research. N. S. W. Public Health Bull. 2009 doi: 10.1071/NB09007. [DOI] [PubMed] [Google Scholar]
- Mwangi Waithaka, de Figueiredo Paul, Criscitiello Michael F. One health: addressing global challenges at the nexus of human, animal, and environmental health. PLoS Pathog. 2016;12(9) doi: 10.1371/journal.ppat.1005731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Ki Chang, Jo Cheorun, Mooha Lee. Meat products and consumption culture in the east. Meat Sci. 2010 doi: 10.1016/j.meatsci.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Nasi R., et al. 2008. 33 Diversity Conservation and Use of Wildlife-Based Resources: The Bushmeat Crisis. [Google Scholar]
- Neumann Charlotte G., et al. Animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children: background, study design and baseline findings. J. Nutr. 2003 doi: 10.1093/jn/133.11.3941S. [DOI] [PubMed] [Google Scholar]
- Nielsen Martin R., et al. The importance of wild meat in the global south. Ecol. Econ. 2018:146. [Google Scholar]
- Ozioko Kingsley Uchenna, Okoye Chris Ikem, Obiezue Rose Nduka, Agbu Raymond Awudu. Knowledge, attitudes, and behavioural risk factors regarding zoonotic infections among bushmeat hunters and traders in Nsukka, Southeast Nigeria. Epidemiol. Health. 2018 doi: 10.4178/epih.e2018025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden C.R., et al. Zoonotic origin and transmission of middle east respiratory syndrome coronavirus in the UAE. Zoonoses Public Health. 2018 doi: 10.1111/zph.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain Deborah J., et al. Potential hazard to human health from exposure to fragments of lead bullets and shot in the tissues of game animals. PLoS One. 2010 doi: 10.1371/journal.pone.0010315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Mirae, Thwaites Ryan S., Openshaw Peter J.M. COVID-19: lessons from SARS and MERS. Eur. J. Immunol. 2020 [Google Scholar]
- Parrish Colin R., et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008;72(3) doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeling Rosanna W., Murtagh Maurine, Olliaro Piero L. Epidemic preparedness: why is there a need to accelerate the development of diagnostics? Lancet Infect. Dis. 2019 doi: 10.1016/S1473-3099(18)30594-2. [DOI] [PubMed] [Google Scholar]
- Peeters Martine, et al. Risk to human health from a plethora of simian immunodeficiency viruses in primate bushmeat. Emerging Infect. Dis. 2002 doi: 10.3201/eid0805.01-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman Stanley. Another decade, another coronavirus. N. Engl. J. Med. 2020 doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philavong Chanfong, et al. Perception of health risks in Lao market vendors. Zoonoses Public Health. 2020 doi: 10.1111/zph.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin Stanley, et al. The complexity and cost of vaccine manufacturing – an overview. Vaccine. 2017 doi: 10.1016/j.vaccine.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright Raina K., et al. Land use-induced spillover: a call to action to safeguard environmental, animal, and human health. Lancet Planet. Health. 2021;5(4):e237–45. doi: 10.1016/S2542-5196(21)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruvot Mathieu, et al. Toward a quantification of risks at the Nexus of conservation and health: the case of bushmeat markets in Lao PDR. Sci. Total Environ. 2019 doi: 10.1016/j.scitotenv.2019.04.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R Core Team (2014). R: A Language and Environment for Statistical Computing.http://www.R-project.org/ URL. [Google Scholar]
- Rewar Suresh, Mirdha Dashrath. Transmission of ebola virus disease: an overview. Ann. Glob. Health. 2014 doi: 10.1016/j.aogh.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Ripple William J., et al. Bushmeat hunting and extinction risk to the world’s mammals. R. Soc. Open Sci. 2016 doi: 10.1098/rsos.160498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts Christine C. Emerging infectious disease laboratory and diagnostic preparedness to accelerate vaccine development. Hum. Vaccin. Immunother. 2019 doi: 10.1080/21645515.2019.1634992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe Dilys, et al. “Beyond banning wildlife trade: COVID-19, conservation and development.”. World Dev. 2020:136. doi: 10.1016/j.worlddev.2020.105121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan M.S., et al. Illegal bushmeat hunters compete with predators and threaten wild herbivore populations in a global tourism hotspot. Biol. Conserv. 2017;210:233–242. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85018329003&doi=10.1016%2Fj.biocon.2017.04.020&partnerID=40&md5=3dc416da241d3a6cf9b55d731f3e44cd [Google Scholar]
- Rogan Matthew S., Miller Jennifer R.B., Lindsey Peter A., Weldon McNutt J. Socioeconomic drivers of illegal bushmeat hunting in a southern african savanna. Biol. Conserv. 2018 [Google Scholar]
- Sans P., Combris P. World meat consumption patterns: an overview of the last fifty years (1961-2011) Meat Sci. 2015 doi: 10.1016/j.meatsci.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Sarti Flavia M., et al. Beyond protein intake: bushmeat as source of micronutrients in the Amazon. Ecol. Soc. 2015 [Google Scholar]
- Saylors Karen E., et al. Market characteristics and zoonotic disease risk perception in Cameroon bushmeat markets. Soc. Sci. Med. 2021:268. doi: 10.1016/j.socscimed.2020.113358. [DOI] [PubMed] [Google Scholar]
- Schilling Megan A., et al. Molecular species identification of bushmeat recovered from the serengeti ecosystem in Tanzania. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237590. (9 September) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Herbrüggen Björn, Cowlishaw Guy, Homewood Katherine, Marcus Rowcliffe J. The importance of bushmeat in the livelihoods of west african cash-crop farmers living in a faunally-depleted landscape. PLoS One. 2013 doi: 10.1371/journal.pone.0072807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiyaboh Enetimi, Izah Sylvester. Bacteriological assessment of a tidal creek receiving slaughterhouse wastes in Bayelsa State, Nigeria. J. Adv. Biol. Biotechnol. 2017 [Google Scholar]
- Sharp Paul M., Hahn Beatrice H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011 doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen Muhammad Adnan, et al. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020 doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoukat Affan, et al. Projecting demand for critical care beds during COVID-19 outbreaks in Canada. CMAJ. 2020 doi: 10.1503/cmaj.200457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Abha Lakshmi, Jamal Saleha, Baba Shanawaz Ahmad, Islam Md.Manirul. Environmental and health impacts from slaughter houses located on the city outskirts: a case study. J. Environ. Prot. 2014 [Google Scholar]
- Smith Katherine F., Acevedo-Whitehouse K., Pedersen A.B. The role of infectious diseases in biological conservation. Anim. Conserv. 2009 [Google Scholar]
- Song Huai Dong, et al. Cross-host evolution of severe acute respiratory syndrome coronavirus in Palm Civet and human. Proc. Natl. Acad. Sci. U. S. A. 2005 doi: 10.1073/pnas.0409608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South Andy. Rworldmap: a new r package for mapping global data. R J. 2011 [Google Scholar]
- Subramanian Melanie. Zoonotic disease risk and the bushmeat trade: assessing awareness among hunters and traders in Sierra Leone. EcoHealth. 2012 doi: 10.1007/s10393-012-0807-1. [DOI] [PubMed] [Google Scholar]
- Tang Xiaolu, et al. On the origin and continuing evolution of SARS-CoV-2. Nat. Sci. Rev. 2020 doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.C.A., Lymbery A.J., Smith A. Parasites, emerging disease and wildlife conservation. Int. J. Parasitol. 2010 doi: 10.1016/j.ijpara.2010.04.009. [DOI] [PubMed] [Google Scholar]
- van Vliet Nathalie, Mbazza Prosper. Recognizing the multiple reasons for bushmeat consumption in urban areas: a necessary step toward the sustainable use of wildlife for food in Central Africa. Hum. Dimens. Wildl. 2011;16(1) [Google Scholar]
- Van Vliet Nathalie. 2011. 60 CBD Bushmeat Liaison Group LiveLihood ALternatives for the UnsustainabLe Use of Bushmeat. [Google Scholar]
- Viana Mafalda, et al. Assembling evidence for identifying reservoirs of infection. Trends Ecol. Evol. 2014;29(5):270–279. doi: 10.1016/j.tree.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacharapluesadee Supaporn, et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-21240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yiqun, Lehane Catherine, Ghebremeskel Kebreab, Crawford Michael A. Modern organic and broiler chickens sold for human consumption provide more energy from fat than protein. Public Health Nutr. 2010 doi: 10.1017/S1368980009991157. [DOI] [PubMed] [Google Scholar]
- Watsa Mrinalini. Rigorous wildlife disease surveillance. Science. 2020;369(6500) doi: 10.1126/science.abc0017. [DOI] [PubMed] [Google Scholar]
- Webster Robert G. Wet Markets - a continuing source of severe acute respiratory syndrome and influenza? Lancet. 2004 doi: 10.1016/S0140-6736(03)15329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl Hana M., et al. Transmission of ebola virus from pigs to non-human primates. Sci. Rep. 2012 doi: 10.1038/srep00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Zoonoses.https://www.who.int/news-room/fact-sheets/detail/zoonoses [Google Scholar]
- World Health Organization . World Health Organization technical report series; 2012. Research Priorities for Zoonoses and Marginalized Infections. [PubMed] [Google Scholar]
- Wu Fan, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020 doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager Paul, Domingo Gonzalo J., Gerdes John. Point-of-care diagnostics for global health. Annu. Rev. Biomed. Eng. 2008 doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- Yusof Mohammed Farouk, et al. Diversity of middle east respiratory syndrome coronaviruses in 109 dromedary camels based on full-genome sequencing, Abu Dhabi, United Arab Emirates. Emerg. Microbes Infect. 2017 doi: 10.1038/emi.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Shuru, Crang Mike, Zeng Guojun. Constructing freshness: the vitality of wet markets in Urban China. Agric. Human Values. 2020;37(1) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.