Figure 3.
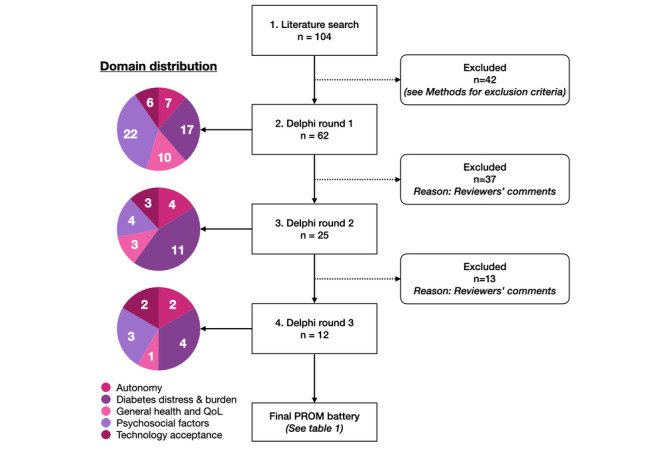
Modified Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram describing the different rounds of the electronic Delphi process and the number of patient-reported outcome measures selected by domain distribution: autonomy, diabetes distress and burden, general health and quality of life, psychosocial factors, and technology acceptance. PROM: patient-reported outcome measure; QoL: quality of life.
