Figure 9. The IFT‐B complex in context of anterograde IFT‐B trains.
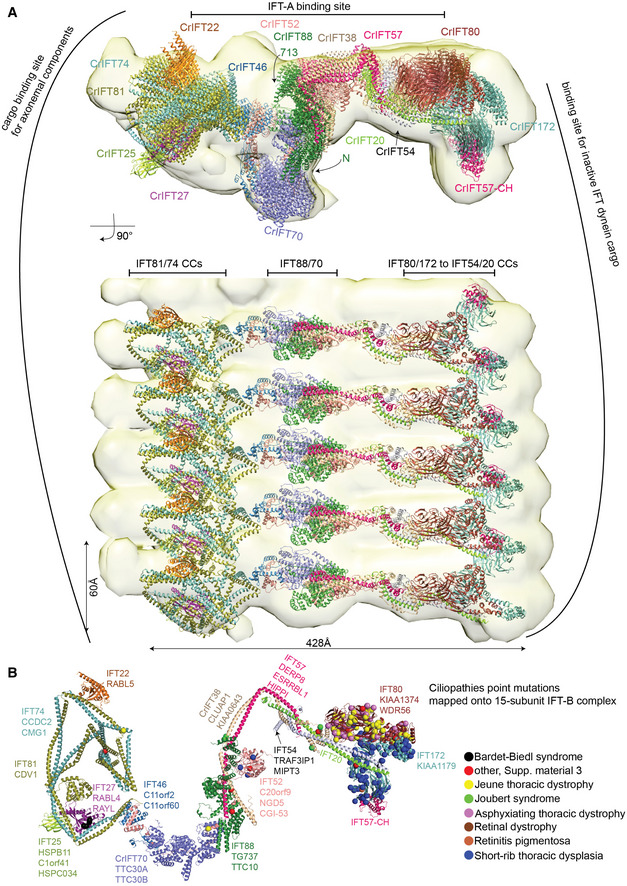
- Molecular dynamic flexible fitting of the IFT‐B 15‐mer into the 25 Å cryo‐electron tomography map of the Chlamydomonas anterograde IFT‐B trains obtained in situ (van den Hoek et al, 2022). The fit is shown in two perpendicular orientations. The highly elongated IFT‐B complex fits with a repeat distance of 60 Å consistent with anterograde IFT trains.
- Single‐point mutations associated with ciliopathies are mapped onto the CrIFT‐B 15‐mer structure as spheres. Noteworthy, mutations that lead to amino acid deletions or additions, as well as frameshifts are not included in the figure. The IFT‐B proteins are annotated according to their corresponding human gene names.
