Abstract

Head and neck squamous cell carcinoma (HNSCC) is the sixth most prevalent cancer in the world and the most prevalent cancer of developing countries. Increased disease burden and a smaller number of approved targeted therapies are a growing concern worldwide. Isoindolinone motifs have been a central part of many pharmacological compounds, and their derivatives possess substantial anticancer potential. However, their anticancer potential against HNSCC has not been well investigated. In the current study, a series of 3-methyleneisoindolinones have been designed and synthesized and their late-stage intramolecular Heck cyclization was achieved to evaluate their anticancer potential against HNSCC cells. Additionally, in silico ADME profiling of synthesized compounds revealed their drug-likeness properties as potential drug candidates. Among the synthesized compounds, 3-bromo-5-methylpyridin-2-yl-3-methyleneisoindolin-1-one, i.e., 3n, with a pyridyl unit exhibited the most significant cytotoxicity against HNSCC cells. The cytotoxic potential of synthesized compounds varied depending on the nature of substituents present and has been well established with structure–activity relationship studies. Further, flow cytometric analysis showed that 3f, 3h, and 3n triggered intracellular oxidative stress, disrupted mitochondrial membrane potential, and interrupted the cell cycle of HNSCC cells in the S-phase and sub-G1 phase. Further, 3f, 3h, and 3n also exhibited pro-apoptotic potential and induced cellular apoptosis in the HNSCC cells. Overall, the findings of this study attributed 3-methyleneisoindolinone chemistry and efficacy evaluation and corroborated their anticancer potential against HNSCC. It will pave the way to further design and optimize novel 3-methyleneisoindolinone as effective antitumor agents, which may provide effective treatment modalities against HNSCC.
1. Introduction
Cancer occurring in the oral cavity, larynx, and pharynx regions is collectively known as head and neck cancer or head and neck squamous cell carcinoma (HNSCC).1 HNSCC is the sixth most common cancer worldwide, and the most common cancer of males in India. It accounts for more than 900,000 cases worldwide annually, with a prevalence of >1.2 million cases and >670,000 mortalities by the year 2040. HNSCC has become a global burden and drastically affects developing countries due to ease in access to tobacco products, which contributes to poor prognosis of the disease as well.2 Among South Asian countries, India accounts for 40% of HNC malignancies with significant morbidity and mortality.3 Since very few noninvasive, safe, and effective treatments are available against HNSCC, up to 60% of treated patients experience recurrence and are unresponsive to subsequent therapeutic interventions.4 Absence of a vast array of specific anticancer agents in HNSCC warrants additional studies in this domain to develop novel therapeutic approaches (effective chemotherapeutic or chemo-radiotherapeutic anticancer agents) with effective antitumor agents.5−7 In spite of conventional treatment modalities like surgery and radiotherapy in HNSCC treatment, there are still copious possibilities to alleviate tumor cell proliferation using novel small molecule anticancer compounds.8
Pharmacological applications and bioavailability of indoles have made them a highly privileged class in heterocyclic chemistry.9 Specifically, anticancer agents possessing an indole framework have gained vital recognition in cancer drug discovery ventures.10 This can be acknowledged with standout examples of FDA-approved anticancer drugs such as vincristine, vinblastine, and panobinostat.11,12 Diversification of the basic indole core, which acts as a pharmacophore, has resulted in establishing a mechanism of action. Therefore, an indole architecture/framework is being used to intrigue novel small molecule inhibitors of protein kinase enzymes, for instance, sigma receptor, PIM kinase, histone deacetylases, DNA topoisomerases, and sirtuins [Figure 1].13,14
Figure 1.

Target-based design of anticancer agents.
Further modification of indoles into indolinones with common hydrogen bonding between a pyrrole ring and C-2 carbonyl represented a very important class of anticancer agents.15
Initially, high-throughput screening of compound libraries for VEGF and PDGF inhibitors resulted in the identification of semaxanib (SU-5416), potentially inhibiting tyrosine autophorylation. Later, this compound was clinically tried for colorectal cancer; however, it was discontinued in Phase III. Further advancement in indole-based anticancer agents leads to the development of SU-6668 and SU-11248 by incorporating propionic acid and (diethylaminoethyl)carmaboyl branching, respectively, on the C-4′ position of semaxanib. SU-11248 exhibited significant cytotoxicity and was approved by FDA for renal and gastrointestinal cancer.16,17 In addition to this series of indolin-2-one derivatives, PHA665752 and SU11274 with sulfonyl group modification at the fifth position bearing a 3-methylene indolin-2-one skeleton entered preclinical trials for anticancer activity and found potential inhibitors of Met tyrosine kinase.18,19 These significant studies concluded the indolin-2-one moiety as a pharmacophore in designing anticancer agents [Figure 2].
Figure 2.
Selected indolinone derivatives as anticancer agents in preclinical or clinical trials.
Inspired from these studies and observations, a novel series of 3-methyleneisoindolinones were designed and synthesized to explore in vitro anticancer activities. Earlier studies have reported anticancer activities of indole and isoindolinone derivatives in various cancer types; however, the efficacy of 3-methyleneisoindolinone has not been well explored in HNSCC.10 Further, the MDM2-P53 axis and Cyclin-D have been reported as major molecular targets of isoindolinones, and interestingly, they were also found to be involved in the onsets of HNSCC by regulating cell cycle and apoptosis.20,21 Since, 3-methyleneisoindolinones were not investigated for their anticancer activity in HNSCC progression earlier, the current study was designed to investigate their potential as anticancer agents, which could open doors for the development of novel and effective anticancer therapeutics in the treatment of HNSCC.
2. Results and Discussion
2.1. Synthesis of 3-Methyleneisoindolinones and Their Intramolecularly Heck-Cyclized Derivatives
Inspired by our previous research study to synthesize 3-methyleneisoindolinone and considering the above findings, herein, we utilize a tin(II) triflate (Sn(OTf)2)-catalyzed reaction of 2-acetylcarboxylic (1) acid with substituted primary amines (2a–2p) for the synthesis of 3-methyleneisoindolin-1-ones (3a–3p) in 47–98% yields.22 In extension to this approach, we further subjected three synthesized 3-methyleneisoindolinones to intramolecular Heck cyclization reactions to obtain five- to seven-membered ring products. Synthesis of a series of 19 examples of highly structurally diverse 3-methyleneisoindolinones was achieved using substituted primary amines such as −Ar, −(Het)Ar, −Bn, and (Het)Bn. Primary amines with hetero-aryl substitutions such as pyridyl, pyrazinyl, benzyl, and aryl moieties containing different halogen atoms were selected as substrates to study the impact of different substitutions on the cytotoxic potential of 3-methyleneisoindolinones. Among the 19 compounds, 16 3-methyleneisoindolinone compounds (3a–3p) were substituted, and three compounds, 4a, 4b, and 4c, were intramolecularly Heck-cyclized products of 3a, 3c, and 3d, respectively. The intramolecular Heck cyclization was carried out using Pd(OAc)2 in catalytic amounts along with K3PO4 and triphelyphosphine in the presence of DMF solvent system, resulting in five-membered (4a), six-membered (4b), and seven-membered (4c) ring cyclized 3-methyleneisoindolinone derivatives in 55–78% yield (Scheme 1).23
Scheme 1. Synthesis of 3-Methyleneisoindolinones and Their Late-Stage Modification via Intramolecular Heck Cyclization.

2.2. In Silico ADME Profiling of Representative Compounds
Since discovering an effective drug-like compound is associated with supreme challenges, several parameters are simultaneously required to be satisfied. Drug-like property evaluation and optimization have been set up as crucial elements of drug discovery advancement.24 Therefore, to study the drug-likeness of the synthesized 3-methyleneisoindolinones, ADME profiling was done using swiss ADME software 87.25 Crucial parameters such as physicochemical properties, lipophilicity, water solubility, pharmacokinetics, and the rule of five obeyance were considered for all the 19 compounds. All representative molecules obey the rule of five and satisfy the candidature to be a drug molecule (Supporting Information).
2.3. Structure–Activity Relationship
As shown in Table 1, among the 19 tested compounds, 12 were found to inhibit head and neck squamous cell carcinoma effectively with cytotoxicity at submicromolar concentration. IC50 values for the effective compounds range from 24.99 to 83.29 μM. Among these cytotoxic compounds, derivatives 3n, 3f, and 3h emerged as the most potent compounds (IC50 = 39.81, 46.93, and 63.96 μM, respectively) in 24 h cytotoxicity assay profiling.
Table 1. In Vitro Cytotoxic Activity of Tested Compounds against the HNSCC Cell Line.

The table presents the structure of the compounds (R1 substitution), their respective percent cytotoxicity, and IC50 values at two different time points of 24 and 48 h calculated after three independent experiments. Here, vinblastine was used as a positive control; ND* means the IC50 value was not determined as the compound does not exert >50% cytotoxicity at the highest concentration (100 μM) and ND# means the value was not determined due to the self-aggregation of compounds at high concentrations that leads to drop in cytotoxicity potential.
A brief investigation study of structure–activity relationships (SARs) discovered that selecting the primary amine with pyranazyl and pyridyl moieties was significant for head and neck squamous cell carcinoma inhibitory activity as deduced by a remarkable increase in the IC50 value elicited by compounds 3f and 3n (Figure 3). Compound 3a with iodo substitution at R1 (aryl) exhibited a cytotoxicity of 60.3% only, but when varied with methoxy group substitution (3b), an increase in cytotoxic potential was observed, i.e., 72.1% with IC50 = 31.24 μM. Considering the benzylic derivatives 3c–3e, it was found that the introduction of bromo benzylamine (3c) led to no head and neck squamous cell carcinoma inhibitory activity. Similarly, introducing the bromo phenylethylamine (3d) shows less cytotoxic potential, i.e., 41.8% at 100 μM concentration in 48 h. Piperonylamine incorporation (3e) does not make much difference but exhibited only 43.6% cytotoxicity at 100 μM concentration in 48 h.
Figure 3.
Structure–activity relationship (SAR) study of 3-methyleneisoindolinone with R1 substitution.
Examining the bromo isoquinoline amine (3h) and bromo quinoline amine (3i) derivatives, compound 3h showed a two-fold increase in cytotoxicity, i.e., 75.5%, as compared to 3i, indicating the remarkable effect of isomerism, whereas the quinoline amine derivative without containing any heteroatom (3g) possessed only 31.2% cytotoxicity. Compounds 3j–3p with hetero pyridyl amine substitutions were found to exhibit significant inhibitory activity. Correlating cytotoxic potential to their structures, it was observed that compound 3n with methyl and bromo substitution at the pyridyl ring showed the most potent inhibitory activity (IC50 = 24.90 μM) among all pyridyl derivatives and in all 19 molecules at 48 h post treatment. Mono chloro substitution (3j) at the pyridyl ring resulted in almost two-fold decrease in inhibitory activity (IC50 = 52.91 μM) when compared to 3n with mono bromo substitution, whereas only 49.3% cytotoxicity was observed when dichloro substitution at the pyridyl ring (3o) was done. Derivative 3p having mono (ortho) chloro substitution at the pyridyl ring possessed only 40.7% cytotoxicity in 48 h [Table 1].
Furthermore, nitrile substitution at the pyridyl ring (3k and 3l) decreases its cytotoxic potential and showed less than 50% cytotoxicity at 48 h. Similarly, another halogen substitution, i.e., iodo (3m), was also not found as a significant inhibitor as it exhibited only 57.1% cytotoxicity. Another series of compounds, 4a–4c, that were intramolecularly Heck-cyclized products of 3a, 3c, and 3d do not significantly inhibit the HNSCC cell line. Compound 4a containing five-membered rings exhibited IC50 = 83.2 μM, whereas 4b and 4c with six- and seven-membered rings, respectively, possessed less than 50% cytotoxicity in 48 h. Conclusively, an increase in ring size was not beneficial for inhibitory activity against the HNSCC cell line. From structure–activity relationship analysis, it could be deduced that substituent variation at the pyridyl moiety of 3-methyleneisoindolinones was significant for the inhibitory activity against HNSCC cells [Table 1].
2.4. Isoindolinone Derivatives Inhibit the Growth of Tongue Squamous Carcinoma Cells
To evaluate the cytotoxic potential of the developed 3-methyleneisoindolinone derivatives on CAL27 cells, an MTT assay26 was performed for 24 and 48 h post-treatment with compounds. Among the pool of 19 derivatives, a significant level of toxic effects compared to the control was observed in most treatment groups (Figure 4). 3a, 4a, 4b, 4c, 3g, 3i, 3n, 3b, 3f, 3h, 3m, and 3o were found to exhibit toxicity throughout the treatment concentration and timeline. However, no significant toxic effect was observed with the rest of the compounds. On some occasions, the cytotoxic effect of compounds was greater at lower concentrations as compared to the higher ones, which was attributed to the crystal formation at higher concentrations. Among this pool, stringent criteria were imposed for the selection of compounds for further assays, i.e., the compound should exhibit dose-dependent inhibition of the cell viability and the compound should display half-maximal inhibitory concentration (IC50) after 24 h of treatment (Table 1). Three compounds with the best IC50 values (3n, 3f, and 3h) were identified and selected for further experiments.
Figure 4.

3-Methyleneisoindolinone derivatives inhibit growth of HNSCC cells. (a–d) Cell cytotoxicity of synthesized 3-methyleneisoindolinone. Results are expressed as mean ± SD (standard deviation) where n = 3. Statistical significance of the data is presented as compared to the control; ns represents that a non-significant difference was observed; *, **, ***, and **** represent statistical significance with P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively.
2.5. 3n, 3f, and 3h Disrupt Mitochondrial Membrane Potential and Trigger Intracellular Oxidative Stress in Cancer Cells
Measurement of intracellular oxidative stress and mitochondrial membrane potential (MMP) is used to evaluate the capability of anticancer compounds to generate cellular stress and disrupt homeostasis of cancer cells.27 Alteration in the levels of intracellular reactive oxygen species (ROS) plays a crucial role in tumorigenesis and tumor development.28 Since the mitochondria are the source of ROS generation in cells, decreased MMP leads to leakage and accumulation of reactive oxygen species (ROS) in cellular compartments. The mitochondria are considered as a target of ROS inside the cells as well. Prominent levels of intracellular oxidative stress are accounted for inverse correlation between the mitochondrial membrane potential (ΔΨm)/(MMP) and generated ROS. The intracellular oxidative stress decreases in the mitochondrial membrane potential (ΔΨm) and eventually leads to the induction of apoptosis in the cells.29 DCFDA-H2 fluorescent dye was used to detect generation of intracellular oxidative stress in the CAL 27 cells post-treatment of the isoindolinone derivatives. DCFDA-H2 reduced to cellular product DCF in the presence of cellular ROS and emitted green fluorescence.
Similarly, cationic dye JC-1 was used to evaluate the mitochondrial membrane potential since it has the property to accumulate in the mitochondria. JC-1 emits red-orange fluorescence due to aggregation of dye in the energized healthy mitochondria (J-aggregates), and it emits green fluorescence when bound to non-energized unhealthy mitochondria (J-monomers) due to decreased mitochondrial membrane potential (MMP). In this study, we observed that compared to the control cells, treatment with isoindolinone derivatives rapidly increases J-monomers (green emission) and decreased J-aggregates (red-emission) in CAL 27 cells (Figure 5a). Cell damage and disturbed MMP were categorized by the ratio of J-aggregates to J-monomers, which was 2.820 for the control group (average value; n = 3) and decreased in a dose-dependent manner post-treatment of 3n (lowest as 0.70 at 75 μM), 3f (lowest as 0.59 at 75 μM), and 3h (lowest as 0.80 at 75 μM) similar to the control drug vinblastine (50 μM) (Figure 5b–d), indicating a decrease in the mitochondrial membrane potential (P < 0.0004).
Figure 5.
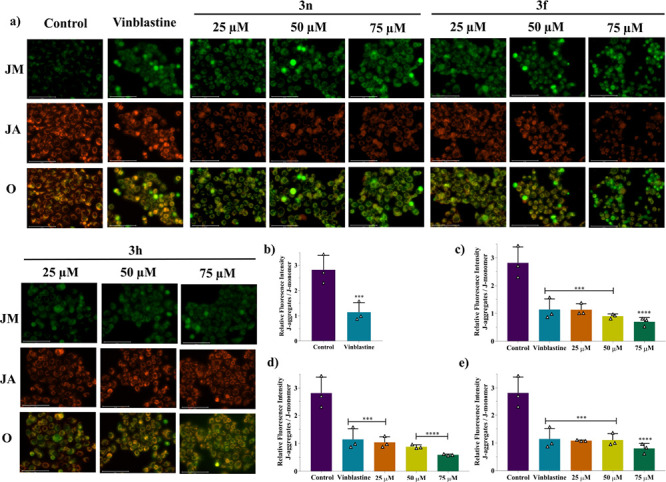
3n, 3f, and 3h disrupt mitochondrial membrane potential (MMP) of HNSCC cells. (a) Fluorescence microscopy images of the disruption of the mitochondrial membrane potential of compounds at different concentrations detected using JC-1 dye. Images are captured at 40× magnification (scalebar: 50 μm) through GFP channel displaying JM (J-monomers), RFP channel displaying JA (J-aggregates), and overlay (O). Graphs representing the decrease in MMP in different treatment groups as compared to the control and along with vinblastine treatment: (b) standard control (vinblastine), (c) 3n, (d) 3f, and (e) 3h. Results are expressed as mean ± SD (n = 3). *** and **** represent statistical significance with P < 0.001 and P < 0.0001, respectively, in comparison to the control group.
Additionally, we also observed the elevated levels of ROS in HNSCC cells treated with 3-methyleneisoindolinone, which is characterized by an increased DFC content (green fluorescence) after treatment of 50 μM of compounds for 4 h (Figure 6). Results clearly indicate that the isoindolinone derivatives have generated intracellular oxidative stress and disrupted the mitochondrial membrane potential (ΔΨm) in the HNSCC cells similar to the potent anticancer compounds in recent studies.30,31 Therefore, it could be characterized as the potential of 3-methyleneisoindolinones to work as potent anticancer agents. However, in order to accentuate the results and delve deeper into the mode of action of the compounds, the effects of the 3-methyleneisoindolinones on key cellular processes like cell cycle and apoptosis were evaluated.
Figure 6.

3n, 3f, and 3h trigger intracellular oxidative stress and ROS production in HNSCC cells. Fluorescence microscopy images depicting ROS generation in CAL 27 cells post-treatment. Images were obtained at 10× magnification (scalebar: 50 μm) through GFP and trans channel and represented in ROS-DCF (GFP), trans, and overlay format.
2.6. 3n, 3f, and 3h Arrest the Cell Cycle of HNSCC Cells in the S-Phase and Sub-G1 Phase
Sustained proliferation and non-regulated cell cycle are two of the major causes of tumorigenesis and the center of the target for anticancer drug development.32−34 Disruption in the cell cycle could lead to a halt in this process and suppress the growth of cancer cells. Flow cytometric analysis was used to characterize the effects of 3n, 3f, and 3h on the cell cycle events of HNSCC cells. Post-treatment, for compound 3n, the cell populations in the S-phase and Sub-G1 phase were increased to 22 and 61% at the highest concentrations as compared to the 10% in control cells, respectively, indicating arrest of the cell cycle in these phases. The presence of the highest percentage of cells in G1 clearly indicates its capability to induce cellular apoptosis as well (Figure 7c). Further, for compound 3f, maximum population (43.8%) was observed in the S-phase, indicating the arrest of the cell cycle in this phase (Figure 7d). Additionally, after treatment of 3h, the highest cell percentage was visible in the G2/M phase (36.3% at 50 μM), exhibiting the arrest (Figure 7e) similar to the vinblastine (50 μM) treatment (Figure 7a). This indicates the ability of 3-methyleneisoindolinone to disturb the cell cycle process, which was in consonance with previous findings of a similar category of anticancer compounds.31,35,36 However, it was interesting to find that all the three leads arrest the cell cycle in different phases and could have a different mode of action on cancer cells. Additionally, a dose-dependent increase in the percentage of the cell population was observed in the Sub-G1 phase for 3n and 3h, indicating the presence of cellular apoptosis (Figure 7c,e). It prompts us to further evaluate the potential of the selected lead compounds to induce cellular apoptosis. Furthermore, these results are in consonance with earlier reports that mentioned isoindolinone derivatives majorly as regulators of Cyclins and CDKs and modulator of PI3K-Akt-dependent G1 arrest.21,37,38 This could be probed further to evaluate Cyclins and CDKs as the molecular targets of these 3-methyleneisoindolinones.
Figure 7.

3n, 3f, and 3h arrest the cell cycle of HNSCC cells in the S-phase and sub-G1 phase. (a) Cell cycle distribution pattern of CAL 27 cells post-treatment of compounds, stained with DAPI and analyzed by flow cytometry. Graphical representation of the cell cycle pattern denoting percent cell populations present in different phases of the cell cycle: (b) standard control (vinblastine), (c) 3n, (d) 3f, and (e) 3h. The dose-dependent arrest of the cell cycle in the S-phase and G2/M phase was observed along with a marked increase in the Sub-G1 population. Results are expressed as mean ± SD (n = 3). **** and *** represent statistical significance with P < 0.0001 and P < 0.001, respectively, in comparison to the control group.
2.7. 3n, 3f, and 3h Possess Pro-apoptotic Potential and Induce Cellular Apoptosis
Apoptosis and its role in tumorigenesis are cornerstones of cell death and disease research in cancer.39 Evasion of apoptosis is a hallmark of cancer cells and one of the major responsible factors for oncogenesis,40 and targeting apoptosis as a cancer therapeutic approach has yielded effective apoptosis inducers as chemotherapeutic drug candidates.41,42 3-Methyleneisoindolinones have induced strong cytotoxic effects; however, the mechanism of the effect was not clear. Also, results obtained from the MMP assay and cell cycle analysis have indicated strong possibilities of 3-methyleneisoindolinone to induce apoptosis. Induction of the cellular apoptosis is often accompanied with reduction in mitochondrial membrane potential (ΔΨm)/(MMP).43 Decreased ΔΨm along with increased reactive oxygen species (ROS) and cellular stress leads to the apoptosis induction. Since our results indicated that isoindolinone treatment increased ROS levels and decreased mitochondrial membrane potential in cancer cells (Figures 5 and 6), therefore, we directly advanced to evaluate the levels of cellular apoptosis in treated cells to confirm the obtained results. Therefore, followed by measurements of ΔΨm and ROS generation, we have used Annexin-V/FITC and PI staining via flow cytometry, which is regarded as a gold standard technique to measure apoptosis in biological system. As depicted in Figure 8, quadrant 3 represents the live cell population (FITC-/PI-), quadrant 1 represents the dead cells (FITC-/PI+), and quadrants 4 and 2 represent the early apoptotic (FITC+/PI-) and late apoptotic cell populations (FITC+/PI+), respectively. Interestingly, treatment of all the compounds increased the early and late apoptotic cell percentage as compared to the control group (P < 0.0001 across the treatment groups) (Figure 8). Results substantiated that a dose-dependent increase in early and late apoptotic cells was observed in 3n and 3h treatment groups (Figure 8c,e), which was in consonance with the cell cycle analysis results (Figure 7).
Figure 8.

3n, 3f, and 3h possess the pro-apoptotic potential and induce cellular apoptosis in HNSCC cells. (a) Dot-plot of the flow cytometric analysis of AnnexinV-FITC/PI-stained cells after treatment of compounds, depicting apoptotic cell population. Here, cells present in Q4 are early (FITC+/PI-) apoptotic cells and those in Q2 are late (FITC+/PI+) apoptotic cells. Bar plots depict the graphical representation of live (Q3; FITC-/PI-), dead (Q1; FITC-/PI+), and apoptotic (Q4 and Q2) cell population percentage after treatment of (b) standard control (vinblastine), (c) 3n, (d) 3f, and (e) 3h. A dose-dependent increase in early and late apoptotic cells was observed in treatment groups. Results are expressed as mean ± SD (n = 3). **** represents statistical significance with P < 0.0001 as compared to the control group.
Additionally, results indicated that, in comparison to the total combined apoptotic cell percentage (early plus late apoptotic cells) after 50 μM vinblastine treatment (45.25), the selected compounds exhibited encouraging results. The percentages of total apoptotic cells for 3n and 3h were found to be 52.08 and 50.11%, respectively, and for 3f, it was a competitive 30.63%. Furthermore, 3h exhibited a lesser percentage of dead cells and more apoptotic cells as compared to vinblastine treatment (Figure 8a,e). It indicates that the compounds exhibit strong pro-apoptotic potential. In summary, the obtained results suggested that the SAR-led changes potentiate the efficacy of 3-methyleneisoindolinone derivatives. Collectively, the results of the study supported that 3-methyleneisoindolinones decrease mitochondrial membrane potential, increase ROS, and disrupt the cell cycle, which leads to the induction of apoptosis in HNSCC cells.
3. Conclusions
In summary, this study depicts the design and synthesis of novel 3-methyleneisoindolinones and their intramolecularly Heck-cyclized derivatives. The compound synthesis was performed in good to excellent yield. It has been observed that 3-methyleneisoindolinones exhibit cytotoxic properties against head and neck squamous cell carcinoma (HNSCC) cells. SAR studies attributed the cytotoxic potential of the synthesized compounds. Synthesized derivatives generated reactive oxygen species (ROS), triggered intracellular oxidative stress, and disrupted the mitochondrial membrane potential (MMP) of CAL27 cells, indicating their strong potential to damage cellular homeostasis of the cancer cells. Additionally, 3-methyleneisoindolinones were also found to substantiate their anticancer activities through inhibition of the cell cycle in the S and sub-G1 phase. Furthermore, Annexin V-FITC/PI-based flow cytometry analysis revealed that the compounds successfully induce cellular apoptosis in HNSCC cells. In silico ADME profiling of representative compounds revealed their drug-likeness properties, and the synthesized compounds fulfill the criteria to be a drug candidate. Further, earlier reports have mentioned isoindolinone derivatives as inhibitors of MDM2-p53 interactions, regulators of Cyclins and CDKs, inhibitors of PARP-1, and modulator of PI3K-Akt-dependent G1 arrest.21,37,38,44,45 The role of tumor suppressor p53 in regulation of cell cycle and apoptosis is well known, and MDM2-p53 axes have been reported to be involved in onsets of HNSCC.46−48 Additionally, Tp53 mutation is one of the most frequent genetic alterations in head and neck squamous cell carcinoma (HNSCC) and results in an accumulation of p53 protein in tumor cells. This makes the MDM2-p53 axis an attractive target point to improve HNSCC therapy by modulation of tumor suppression activities and apoptosis. Our results have shown that 3-methyleneisoindolinone regulates the cell cycle and increase sub-G1 population, disrupts MMP, and finally induces cellular apoptosis. This makes the synthesized 3-methyleneisoindolinone promising leads against the discussed targets MDM2, p53, and Cyclins, and further studies in this direction would help to delve deeper into mechanistic insights of the compounds in HNSCC. Overall, this study attributes the anticancer potential of novel 3-methyleneisoindolinone and opens doors for further studies pertaining to the mode of action in preclinical models, which may provide effective treatment modalities against HNSCC.
4. Experimental Section
Detailed experimental section is provided in the Supporting Information.
Acknowledgments
Authors are grateful to the Director, CSIR-Institute of Himalayan Bioresource Technology (CSIR-IHBT), Palampur, India for providing the necessary facilities. A.S. (IF170286) and P.A. (IF170140) are thankful to the Department of Science and Technology, Government of India for providing the DST-INSPIRE fellowship. P.A. gratefully acknowledges the Academy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India for his Ph.D. registration. The CSIR-IHBT communication number is 5078.
Glossary
Abbreviations
- CCR2
CC chemokine receptor 2
- CCL2
CC chemokine ligand 2
- CCR5
CC chemokine receptor 5
- DCFH-DA
2′,7′-dichlorofluorescein diacetate
- FITC
fluorescein isothiocyanate
- H2-DCFDA
2′,7′-dichlorofluorescin diacetate
- HNSCC
head and neck squamous cell carcinoma
- MMP
mitochondrial membrane potential
- TLC
thin-layer chromatography
- ROS
reactive oxygen species
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c05378.
Detailed experimental section, 1H NMR and 13C NMR spectra, and UPLC traces of the synthesized compounds (PDF)
Author Present Address
∞ Department of Chemistry, University of Lucknow, Lucknow 226007, India
Author Contributions
# A.S. and P.A. contributed equally to the manuscript.
Author Contributions
S.K.M. and Y.S.P. conceptualized the study. S.K.M. and A.S. designed the chemistry and synthesis part. Y.S.P. and P.A. designed the in vitro experiments. A.S. and P.A. performed the bench experiments and generated the datasets. A.S., P.A., Y.S.P., and S.K.M. analyzed the dataset. A.S. and P.A. wrote the manuscript. Y.S.P. and S.K.M. edited the manuscript. All authors approved the final version of the manuscript.
MLP-203, MLP-0204, and MLP-0155 in house projects funded by CSIR.
The authors declare no competing financial interest.
Dedication
The article is dedicated to Dr. Mukund K. Gurjar on the occasion of his 70th birthday.
Supplementary Material
References
- Elliott D. A.; Nabavizadeh N.; Hiluf K.; Holland J. M. Head and Neck Cancer. Med. Radiol. 2017, 137–157. 10.1007/174_2017_47. [DOI] [Google Scholar]
- Johnson D. E.; Burtness B.; Leemans C. R.; Lui V. W. Y.; Bauman J. E.; Grandis J. R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 1–22. 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S. S.; Raksha Rao K.; Jain A.; Bawa P. S.; Dutta P.; Atre G.; Subhash A.; Rao V. U. S.; Suvratha J.; Srinivasan S.; Choudhary B.. Multidimensional Mutational Profiling of the Indian HNSCC Sub-Population Provides IRAK1, a Novel Driver Gene and Potential Druggable Target. Front. Oncol. 2021, 11, 10.3389/FONC.2021.723162/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer J. D.; Burtness B.; Le Q. T.; Ferris R. L. The Changing Therapeutic Landscape of Head and Neck Cancer. Nat. Rev. Clin. Oncol 2019, 16, 669–683. 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- Kozakiewicz P.; Grzybowska-Szatkowska L. Application of Molecular Targeted Therapies in the Treatment of Head and Neck Squamous Cell Carcinoma. Oncol. Lett. 2018, 15, 7497–7505. 10.3892/ol.2018.8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstonog G.; Simon C. Trends in Surgical Research in Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 1–13. 10.1007/S11864-017-0475-Z. [DOI] [PubMed] [Google Scholar]
- Chen J.; Li Q.; Wang F.; Yang M.; Xie L.; Zeng X. Biosafety, Nontoxic Nanoparticles for VL-NIR Photothermal Therapy against Oral Squamous Cell Carcinoma. ACS Omega 2021, 6, 11240–11247. 10.1021/ACSOMEGA.1C00101/ASSET/IMAGES/LARGE/AO1C00101_0006.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsahafi E.; Begg K.; Amelio I.; Raulf N.; Lucarelli P.; Sauter T.; Tavassoli M. Clinical Update on Head and Neck Cancer: Molecular Biology and Ongoing Challenges. Cell Death Dis. 2019, 10, 1–17. 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.; Li Y.; Yan C.; Yan M.; Tang Z. Indole: A Privileged Scaffold for the Design of Anti-Cancer Agents. Eur. J. Med. Chem. 2019, 183, 111691 10.1016/j.ejmech.2019.111691. [DOI] [PubMed] [Google Scholar]
- Dadashpour S.; Emami S. Indole in the Target-Based Design of Anticancer Agents: A Versatile Scaffold with Diverse Mechanisms. Eur. J. Med. Chem. 2018, 150, 9–29. 10.1016/j.ejmech.2018.02.065. [DOI] [PubMed] [Google Scholar]
- Hong Y.; Zhu Y. Y.; He Q.; Gu S. X. Indole Derivatives as Tubulin Polymerization Inhibitors for the Development of Promising Anticancer Agents. Bioorg. Med. Chem. 2022, 55, 116597 10.1016/j.bmc.2021.116597. [DOI] [PubMed] [Google Scholar]
- Kumari A.; Singh R. K. Medicinal Chemistry of Indole Derivatives: Current to Future Therapeutic Prospectives. Bioorg. Chem. 2019, 89, 103021 10.1016/j.bioorg.2019.103021. [DOI] [PubMed] [Google Scholar]
- Chadha N.; Silakari O. Indoles as Therapeutics of Interest in Medicinal Chemistry: Bird’s Eye View. Eur. J. Med. Chem. 2017, 134, 159–184. 10.1016/j.ejmech.2017.04.003. [DOI] [PubMed] [Google Scholar]
- R Prakash C.; Raja S. Indolinones as Promising Scaffold as Kinase Inhibitors: A Review. Mini-Rev. Med. Chem. 2012, 12, 98–119. 10.2174/138955712798995039. [DOI] [PubMed] [Google Scholar]
- El-Sharief A. M. S.; Ammar Y. A.; Belal A.; El-Sharief M. A. M. S.; Mohamed Y. A.; Mehany A. B. M.; Elhag Ali G. A. M.; Ragab A. Design, Synthesis, Molecular Docking and Biological Activity Evaluation of Some Novel Indole Derivatives as Potent Anticancer Active Agents and Apoptosis Inducers. Bioorg. Chem. 2019, 85, 399–412. 10.1016/j.bioorg.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Laird A. D.; Vajkoczy P.; Shawver L. K.; Thurnher A.; Liang C.; Mohammadi M.; Schlessinger J.; Ullrich A.; Hubbard S. R.; Blake R. A.; Fong T. A. T.; Strawn L. M.; Sun L.; Tang C.; Hawtin R.; Tang F.; Shenoy N.; Hirth K. P.; McMahon G.; Cherrington J. M. SU6668 Is a Potent Antiangiogenic and Antitumor Agent That Induces Regression of Established Tumors. Cancer Res. 2000, 60, 4152–4160. [PubMed] [Google Scholar]
- Abrams T. J.; Murray L. J.; Pesenti E.; Holway V. W.; Colombo T.; Lee L. B.; Cherrington J. M.; Pryer N. K. Preclinical Evaluation of the Tyrosine Kinase Inhibitor SU11248 as a Single Agent and in Combination with “Standard of Care” Therapeutic Agents for the Treatment of Breast Cancer. Mol. Cancer Ther. 2003, 2, 1011–1021. [PubMed] [Google Scholar]
- Sattler M.; Pride Y. B.; Ma P.; Gramlich J. L.; Chu S. C.; Quinnan L. A.; Shirazian S.; Liang C.; Podar K.; Christensen J. G.; Salgia R. A Novel Small Molecule Met Inhibitor Induces Apoptosis in Cells Transformed by the Oncogenic TPR-MET Tyrosine Kinase. Cancer Res. 2003, 63, 5462–5469. [PubMed] [Google Scholar]
- Christensen J. G.; Schreck R.; Burrows J.; Kuruganti P.; Chan E.; Le P.; Chen J.; Wang X.; Ruslim L.; Blake R.; Lipson K. E.; Ramphal J.; Do S.; Cui J. J.; Cherrington J. M.; Mendel D. B. A Selective Small Molecule Inhibitor of C-Met Kinase Inhibits c-Met-Dependent Phenotypes in Vitro and Exhibits Cytoreductive Antitumor Activity in Vivo. Cancer Res. 2003, 63, 7345–7355. [PubMed] [Google Scholar]
- de Bakker T.; Journe F.; Descamps G.; Saussez S.; Dragan T.; Ghanem G.; Krayem M.; Van Gestel D. Restoring P53 Function in Head and Neck Squamous Cell Carcinoma to Improve Treatments. Front. Oncol. 2022, 11, 5665. 10.3389/FONC.2021.799993/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessari G.; Hardcastle I. R.; Ahn J. S.; Anil B.; Anscombe E.; Bawn R. H.; Bevan L. D.; Blackburn T. J.; Buck I.; Cano C.; Carbain B.; Castro J.; Cons B.; Cully S. J.; Endicott J. A.; Fazal L.; Golding B. T.; Griffin R. J.; Haggerty K.; Harnor S. J.; Hearn K.; Hobson S.; Holvey R. S.; Howard S.; Jennings C. E.; Johnson C. N.; Lunec J.; Miller D. C.; Newell D. R.; Noble M. E. M.; Reeks J.; Revill C. H.; Riedinger C.; St. Denis J. D.; Tamanini E.; Thomas H.; Thompson N. T.; Vinković M.; Wedge S. R.; Williams P. A.; Wilsher N. E.; Zhang B.; Zhao Y. Structure-Based Design of Potent and Orally Active Isoindolinone Inhibitors of MDM2-P53 Protein-Protein Interaction. J. Med. Chem. 2021, 64, 4071–4088. 10.1021/ACS.JMEDCHEM.0C02188/SUPPL_FILE/JM0C02188_SI_003.CIF. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Nayal O. S.; Sharma A.; Rana R.; Maurya S. K. Tin(II) Triflate Catalysed Synthesis of 3-Methyleneisoindolin-1-Ones. ChemistrySelect 2019, 4, 1985–1988. 10.1002/slct.201804009. [DOI] [Google Scholar]
- Li Z.; Liang Y.; Zhu Y.; Tan H.; Li X.; Wang W.; Zhang Z.; Jiao N.. 3.02 - Prroles and Their Benzo Derivatives: Reactivity. In Comprehensive Heterocyclic Chemistry IV; Black D. S.; Cossy J.; Stevens C. V. Eds.; Elsevier: Oxford, 2022; pp. 68–155, 10.1016/B978-0-12-409547-2.14853-X. [DOI] [Google Scholar]
- Ferreira L. L. G.; Andricopulo A. D. ADMET Modeling Approaches in Drug Discovery. Drug Discovery Today 2019, 24, 1157–1165. 10.1016/j.drudis.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Daina A.; Michielin O.; Zoete V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 1–13. 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meerloo J.; Kaspers G. J. L.; Cloos J. Cell Sensitivity Assays: The MTT Assay. Methods Mol. Biol. 2011, 731, 237–245. 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- Gottlieb E.; Armour S. M.; Harris M. H.; Thompson C. B. Mitochondrial Membrane Potential Regulates Matrix Configuration and Cytochrome c Release during Apoptosis. Cell Death Differ. 2003, 10, 709–717. 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- Cheung E. C.; Vousden K. H. The Role of ROS in Tumour Development and Progression. Nat. Rev. Cancer 2022, 22, 280–297. 10.1038/s41568-021-00435-0. [DOI] [PubMed] [Google Scholar]
- Redza-Dutordoir M.; Averill-Bates D. A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta, Mol. Cell Res. 2016, 1863, 2977–2992. 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Qin J.; Sun X.; Ma Y.; Cheng Y.; Ma Q.; Jing W.; Qu S.; Liu L. Design, Synthesis and Biological Evaluation of Novel 1,3,4,9-Tetrahydropyrano[3,4-b]Indoles as Potential Treatment of Triple Negative Breast Cancer by Suppressing PI3K/AKT/MTOR Pathway. Bioorg. Med. Chem. 2022, 55, 116594 10.1016/j.bmc.2021.116594. [DOI] [PubMed] [Google Scholar]
- Yan J.; Xu Y.; Jin X.; Zhang Q.; Ouyang F.; Han L.; Zhan M.; Li X.; Liang B.; Huang X. Structure Modification and Biological Evaluation of Indole-Chalcone Derivatives as Anti-Tumor Agents through Dual Targeting Tubulin and TrxR. Eur. J. Med. Chem. 2022, 227, 113897 10.1016/j.ejmech.2021.113897. [DOI] [PubMed] [Google Scholar]
- Hanahan D.; Weinberg R. A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Diaz-Moralli S.; Tarrado-Castellarnau M.; Miranda A.; Cascante M. Targeting Cell Cycle Regulation in Cancer Therapy. Pharmacol. Ther. 2013, 138, 255–271. 10.1016/j.pharmthera.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Petroni G.; Formenti S. C.; Chen-Kiang S.; Galluzzi L. Immunomodulation by Anticancer Cell Cycle Inhibitors. Nat. Rev. Immunol. 2020, 20, 669–679. 10.1038/s41577-020-0300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan K.; Satyanarayana V. S. V.; Sivakumar A. Synthesis and Biological Evaluation of Novel Phenothiazine Derivatives as Potential Antitumor Agents. Polycyclic Aromat. Compd. 2022, 46, 5970–5977. 10.1080/10406638.2021.2021254. [DOI] [Google Scholar]
- He Z. X.; Huo J. L.; Gong Y. P.; An Q.; Zhang X.; Qiao H.; Yang F. F.; Zhang X. H.; Jiao L. M.; Liu H. M.; Ma L. Y.; Zhao W. Design, Synthesis and Biological Evaluation of Novel Thiosemicarbazone-Indole Derivatives Targeting Prostate Cancer Cells. Eur. J. Med. Chem. 2021, 210, 112970 10.1016/j.ejmech.2020.112970. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Wu J.; Wang J.; Xiao S.; Zhao L.; Yan R.; Wu X.; Wang Z.; Fan L.; Jin Y. Novel Isoindolinone-Based Analogs of the Natural Cyclic Peptide Fenestin A: Synthesis and Antitumor Activity. ACS Med. Chem. Lett. 2022, 13, 1118–1124. 10.1021/ACSMEDCHEMLETT.2C00149/ASSET/IMAGES/LARGE/ML2C00149_0004.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan K.; Elancheran R.; Divakar S.; Anand S. A. A.; Ramanathan M.; Kotoky J.; Lokanath N. K.; Kabilan S. Design, Synthesis and Biological Evaluation of 2-(4-Phenylthiazol-2-Yl) Isoindoline-1,3-Dione Derivatives as Anti-Prostate Cancer Agents. Bioorg. Med. Chem. Lett. 2017, 27, 1199–1204. 10.1016/J.BMCL.2017.01.065. [DOI] [PubMed] [Google Scholar]
- Cotter T. G. Apoptosis and Cancer: The Genesis of a Research Field. Nat. Rev. Cancer 2009, 9, 501–507. 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- Singh P.; Lim B. Targeting Apoptosis in Cancer. Curr. Oncol. Rep. 2022, 24, 273–284. 10.1007/s11912-022-01199-y. [DOI] [PubMed] [Google Scholar]
- Anand P.; Soni S.; Padwad Y. S. Targeted Molecular Therapies in Cancer. Mod. Cancer Ther. Tradit. Med. An Integr. Approach to Combat Cancers 2021, 59–101. 10.2174/9789814998666121010007. [DOI] [Google Scholar]
- Momcilovic M.; Jones A.; Bailey S. T.; Waldmann C. M.; Li R.; Lee J. T.; Abdelhady G.; Gomez A.; Holloway T.; Schmid E.; Stout D.; Fishbein M. C.; Stiles L.; Dabir D. V.; Dubinett S. M.; Christofk H.; Shirihai O.; Koehler C. M.; Sadeghi S.; Shackelford D. B. In Vivo Imaging of Mitochondrial Membrane Potential in Non-Small-Cell Lung Cancer. Nature 2019, 575, 380–384. 10.1038/s41586-019-1715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedinger C.; Endicott J. A.; Kemp S. J.; Smyth L. A.; Watson A.; Valeur E.; Golding B. T.; Griffin R. J.; Hardcastle I. R.; Noble M. E.; McDonnell J. M. Analysis of Chemical Shift Changes Reveals the Binding Modes of Isoindolinone Inhibitors of the MDM2-P53 Interaction. J. Am. Chem. Soc. 2008, 130, 16038–16044. 10.1021/JA8062088/SUPPL_FILE/JA8062088_SI_002.PDF. [DOI] [PubMed] [Google Scholar]
- Papeo G.; Orsini P.; Avanzi N. R.; Borghi D.; Casale E.; Ciomei M.; Cirla A.; Desperati V.; Donati D.; Felder E. R.; Galvani A.; Guanci M.; Isacchi A.; Posteri H.; Rainoldi S.; Riccardi-Sirtori F.; Scolaro A.; Montagnoli A. Discovery of Stereospecific PARP-1 Inhibitor Isoindolinone NMS-P515. ACS Med. Chem. Lett. 2019, 10, 534–538. 10.1021/ACSMEDCHEMLETT.8B00569/ASSET/IMAGES/LARGE/ML-2018-00569D_0006.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann A.; Patel A. A.; Silver N. L.; Tang L.; Liu Z.; Wang L.; Tanaka N.; Rao X.; Takahashi H.; Maduka N. K.; Zhao M.; Chen T. C.; Liu W. W.; Gao M.; Wang J.; Frank S. J.; Hittelman W. N.; Mills G. B.; Myers J. N.; Osman A. A. COTI-2, a Novel Thiosemicarbazone Derivative, Exhibits Antitumor Activity in HNSCC through P53-Dependent and -Independent Mechanisms. Clin. Cancer Res. 2019, 25, 5650–5662. 10.1158/1078-0432.CCR-19-0096/74854/AM/COTI-2-A-NOVEL-THIOSEMICARBAZONE-DERIVATIVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M.; Kondo S.; Shimizu Y.; Wakisaka N.; Murono S.; Furukawa M.; Yoshizaki T. Impact of MDM2 Single Nucleotide Polymorphism on Tumor Onset in Head and Neck Squamous Cell Carcinoma. Acta Oto-Laryngol. 2009, 128, 808–813. 10.1080/00016480701724904. [DOI] [PubMed] [Google Scholar]
- Mantovani F.; Collavin L.; Del Sal G. Mutant P53 as a Guardian of the Cancer Cell. Cell Death Differ. 2018, 26, 199–212. 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




