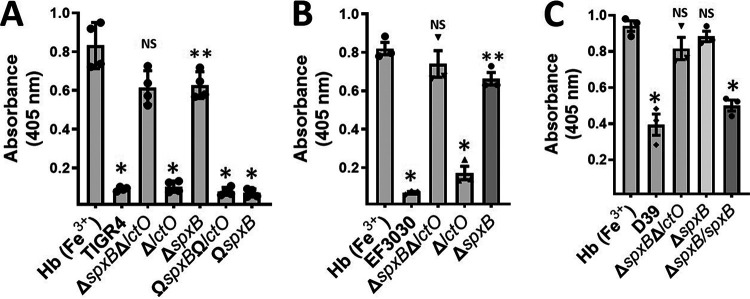
FIG 3.
Oxidation of hemoglobin occurs through hydrogen peroxide produced by SpxB activity but not LctO. (A) Strains TIGR4, TIGR4ΔspxBΔlctO, TIGR4ΔspxB, TIGR4ΔlctO, TIGR4ΩspxBΩlctO, and TIGR4ΩspxB, (B) strains EF3030, EF3030ΔspxBΔlctO, EF3030ΔspxB, and EF3030ΔlctO, and (C) strains D39 and D39ΔspxBΔlctO were inoculated into THY containing methemoglobin [Hb (Fe3+)], and cultures were incubated for 4 h at 37°C in a 5% CO2 atmosphere. Supernatants were collected, and the spectra were obtained using an Omega spectrophotometer (BMG LabTech). The absorbance at 405 nm obtained from each strain was utilized to construct the graphics. Error bars in all panels represent the standard errors of the means calculated using data from at least three independent experiments performed with two technical replicates each. One-way ANOVA with Dunnett’s test for multiple comparison was performed. NS, not significant compared to THY-Hb (Fe3+); *, P < 0.0001 compared with untreated THY-Hb (Fe3+) or the ΔspxB ΔlctO or ΔspxB strain; **, P < 0.003 compared with untreated THY-Hb (Fe3+).