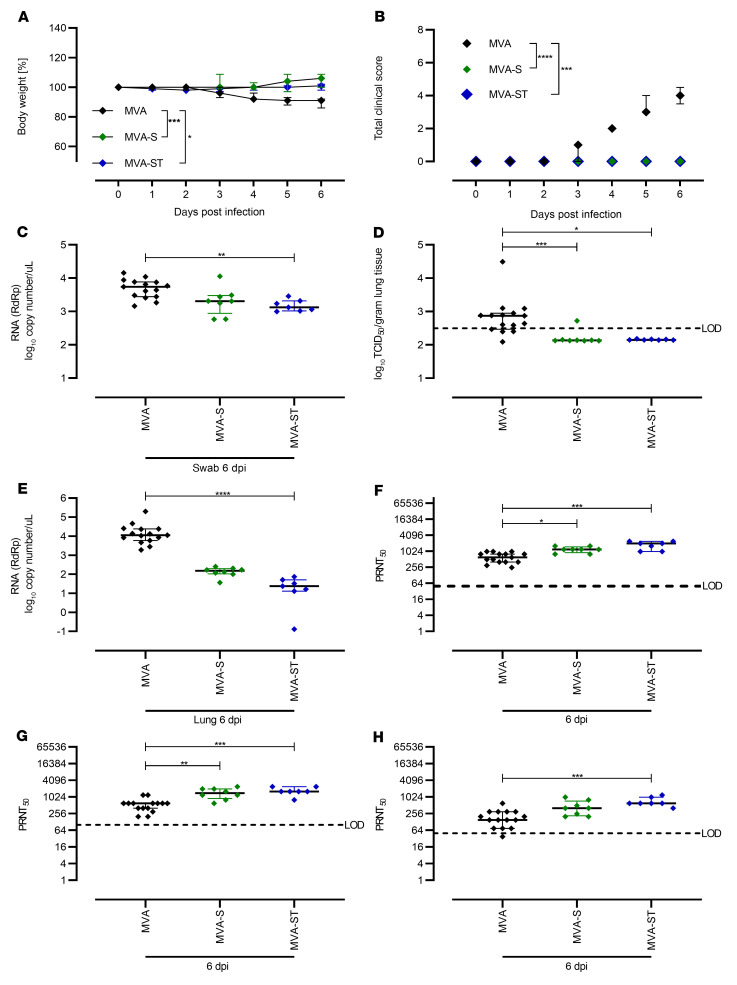
Figure 7. Protective capacity of MVA-S or MVA-ST immunization against SARS-CoV-2 BavPat1 infection in Syrian hamsters.
Syrian hamsters vaccinated with MVA (n = 15) control, MVA-S (n = 8), or MVA-ST (n = 7) were i.n. challenged with 1 × 104 TCID50 SARS-CoV-2 BavPat1. (A) Body weight was monitored daily and (B) spontaneous behavior and general condition were evaluated by clinical scores. (C) Oropharyngeal swabs on day 6 after challenge infection were analyzed for SARS-CoV-2 gRNA copies. (D and E) Lungs were harvested and analyzed for (D) infectious SARS-CoV-2 by TCID50/gram lung tissue, and (E) SARS-CoV-2 gRNA copies. Sera were prepared on day 6 after challenge and analyzed for SARS-CoV-2 (F) BavPat1, (G) Delta, and (H) Omicron variant–neutralizing antibodies by PRNT50. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by Kruskal-Wallis test with Dunn’s multiple comparisons test (C–H) of AUC (A and B). LOD, limit of detection.