Abstract
Rift Valley fever virus (RVFV) is an economically devastating, zoonotic arbovirus endemic across Africa with potential to cause severe disease in livestock and humans. Viral spread is primarily driven by movement of domestic ruminants and there is a high potential for transboundary spread. Despite influx of livestock to urban areas in response to the high demand for meat and animal products, RVFV has not been detected in any urban center. The objectives of this study were to determine the feasibility of assessing risk of RVFV introduction to urban Kisumu, Kenya, by testing slaughtered livestock for RVFV exposure and mapping livestock origins. Blood was collected from cattle, sheep, and goats directly after slaughter and tested for anti-RVFV IgG antibodies. Slaughterhouse businessmen responded to a questionnaire on their individual animals' origin, marketplace, and transport means. Thereafter, we mapped livestock flow from origin to slaughterhouse using participatory methods in focus group discussions with stakeholders. Qualitative data on route choice and deviations were spatially integrated into the map. A total of 304 blood samples were collected from slaughtered livestock in October and November 2021. Most (99%) of animals were purchased from 28 different markets across eight counties in Western Kenya. The overall RVFV seroprevalence was 9% (19% cattle, 3% in sheep, and 7% in goats). Migori County bordering Tanzania had the highest county-level seroprevalence (34%) and 80% of all seropositive cattle were purchased at the Suba Kuria market in Migori County. Road quality and animal health influenced stakeholders' decisions for choice of transport means. Overall, this proof-of-concept study offers a sampling framework for RVFV that can be locally implemented and rapidly deployed in response to regional risk. This system can be used in conjunction with participatory maps to improve active livestock surveillance and monitoring of RVFV in Western Kenya, and these methods could be extrapolated to other urban centers or livestock diseases.
Keywords: RVFV epidemiology, Urban RVFV, Rift Valley fever virus transmission, Urban zoonoses, Urban vector-borne disease, Livestock movement, Slaughterhouse surveillance
Abbreviations: RVFV, Rift Valley fever virus
Highlights
-
•
Influx of livestock to urban slaughterhouses increases risk of urban RVFV introduction.
-
•
Testing urban slaughtered animals for RVFV is feasible and provides animal origin data.
-
•
A 9% overall RVFV seroprevalence, 18% in cattle, in urban slaughtered ruminants in Kisumu, Kenya
-
•
Participatory mapping provides critical context to interpret animal origin data and movement.
-
•
Active surveillance is key to identify RVFV circulation in adult animals that enter urban centers.
1. Introduction
Rift Valley fever virus (RVFV) is an economically devastating livestock disease and a globally important public health threat [1,2]. RVFV is transmitted to humans by arthropod vectors or directly via contact with fluids and tissues of infected livestock [1]. There are currently no human vaccines available and public health control measures rely heavily on management of infection in livestock, with cattle, sheep, and goats being the most affected species [3]. When RVFV enters a naïve herd, it causes a near 100% abortion rate in pregnant animals and death of young animals, and this greatly impacts human livelihoods that rely on livestock to generate income. Over the natural course of an outbreak, infected vectors emerge and RVFV amplifies for several weeks in domestic ruminants before eventually spilling over to humans [4]. A proportion of infected humans will go on to develop severe disease which manifests as encephalitis, hemorrhage, blindness, miscarriage, or even death [5]. Thus, there are economic and health benefits to identifying livestock infections rapidly and improving our understanding of the basic epidemiology of endemic RVFV transmission can guide risk assessment in naïve locations.
RVFV outbreaks have recently become more frequent across Africa and are occurring in new locations, particularly in East Africa, where human cases have been detected yearly since 2016 [6]. In this setting, small holder farming dominates meat value chains, and consumers in urban centers represent a large proportion of this demand as they tend to have more wealth and purchasing power [7]. Yet, urban populations own fewer ruminants and therefore, cattle, sheep, and goats enter urban centers from a wide geographical range to be slaughtered in arrangements carried out privately between livestock traders and local butcheries that sell fresh meat daily. Despite this high volume of livestock movement, there has never been an urban outbreak of RVFV [1]. A previous qualitative study in urban Kenya characterizing risk of introduction into urban areas identified that these imported animals wait in holding pens where they can share vectors with local urban livestock or mix with them while grazing [8]. Legislation in Kenya mandates each slaughterhouse have a meat inspector veterinarian to officially stamp approved meat prior to releasing it from the slaughterhouse [9]. However, previous studies have documented regulatory violations including lack of worker PPE (personal protective equipment), less than ideal infrastructure, and slaughtering of sick animals as inspection professionals often have overwhelming workloads and lack of access to confirmatory diagnostic tools [10]. Despite RVF disease causing severe herd-level clinical signs, adult cattle, the primary candidates for slaughter, typically do not display any clinical signs [11]. Furthermore, the animals that display these classic RVFV clinical signs, pregnant (abortions) and young animals (death), are often not present in the slaughterhouse environment which highlights a critical role for active surveillance to accurately classify RVFV risk. Sampling efforts for live animals are further hampered by the challenging logistics of gathering livestock for medical procedures without safety equipment. This was highlighted in a recent qualitative study that aimed to understand the barriers to vaccinating cattle in Kenya and Uganda, and the authors identified lack of equipment to be a major constraint to gathering cattle for vaccination, and vaccination requires less physical restraint than venipuncture for RVFV testing [12]. Persistence of these livestock blood sampling challenges are manifested by the lack of longitudinal surveillance data which results in missed infections and opportunities to fill gaps in RVFV epidemiology [6,13].
Various arboviruses have established localized urban transmission cycles that are driven by urban vectors as urban areas often have a higher density of vectors particularly, Aedes spp, which are critical for yellow fever virus and Dengue virus, but not yet shown to be key vectors in RVFV transmission [14,15]. Overall, there is a lack of literature examining the potential of urban RVFV and recently, a peri urban outbreak of RVFV was retrospectively identified in Northern Tanzania which borders Kenya to the South [16]. Direct exposure risks to humans have been associated with severe RVF disease and these exposures can be measured in surveys more easily than vector exposures. [17] In urban Kisumu, consumption of animal products was identified as a risk factor independent of livestock ownership [32]. The qualitative component of this study also explained that blood was collected from the Kisumu slaughterhouses daily in containers and was sold independently to buyers that either use it for making local sausage or sell the blood as medicine where it is consumed fresh and uncooked [8]. Consumption of fresh blood is a historical cultural practice in some areas of Kenya and risk of disease may differ in new settings such as urban centers where animals are sourced from many different regions. The origin location of slaughtered animals sourced in urban Kisumu is not systematically tracked and livestock movements across Africa are complex. However, a recent study in rural Western Kenya identified livestock movements to remain relatively static between seasons and defined mixed methods to describe and validate these movements [18]. The main reason that live animals are moved towards urban centers in Kenya is to be slaughtered, and maps that spatially represent this human-driven process might provide valuable insight on disease spread. In understanding how livestock movement impacts disease risk, it is imperative that movement maps are not only created by a few select dominant figures in society as they may misrepresent historic structural inequalities in livestock systems. Maps created in collaboration with key stakeholders of a system represent a socially or culturally distinct understanding of landscape and provide information, such as local knowledge and community-defined boundaries, that is not present in typical maps and simultaneously empower people that operate within the landscape [19].
We aim to alleviate the complicated logistics of live animal blood sampling and costly mobilization of veterinary services by developing a novel blood sampling framework based at slaughterhouses and integrate livestock movement data collected in collaboration with slaughterhouse livestock traders. This study aims to determine the feasibility of testing blood from livestock directly after slaughter and collecting basic epidemiological information on each sample. The impact of this study is intended to classify risk of urban RVFV introduction from different source locations along livestock transport networks in Western Kenya and develop an approach for wide-spread surveillance that operates within the existing slaughterhouse structure.
2. Methods
This study leverages business practices of urban slaughterhouses in Kenya where animals move from rural locations to serve the urban meat market. Our study design had two components: Cross-sectional blood sampling at the slaughterhouse and participatory mapping of supplying origins. The blood sampling component was carried out in October and November 2021, where individual blood samples were collected from animals directly after their slaughter. Thereafter, in January 2022, we gathered stakeholders that purchase animals for slaughter in focus group discussions (FGDs) and collected livestock origins and routes as spatial data using participatory mapping methods. Both components of this study were carried out in Kisumu, Kenya.
Kisumu (0.09171° S, 34.7671° E) is only 11 km south of the equator and has an average temperature of 23 °C with rainfall ranging between 1100 and 1500 mm per year. Kisumu is located adjacent to Lake Victoria at an altitude of 1160 m. It is the largest city in Western Kenya with a population of 1,224,531 people of which 40% reside in informal settlements [20]. A previous community cohort study in Kenya identified a 2% RVFV community exposure rate of RVFV, and identified local animal ownership of ruminants for meat to be <1 % (Stanford IRB: IRB-57869, Technical University of Mombasa IRB: TUM ERC EXT/004/2019R) (Gerken, 2022). Western Kenya was recently reported to be at risk of becoming a new RVFV hotspot in a 2022 early warning system alert initiated by the Food Animal Organization for East Africa (FAO) [21].
2.1. Ethical considerations
This study was approved by Stanford University Institute Review Board (FWA00000935/SU) and Kenya Medical Research Institute Scientific and Ethics Review Unit (SERU).
(KEMRI/SERU/CGHR/03–07-390/4293). A research license in Kenya was obtained from National Commission for Science, Technology & Innovation (License No: NACOSTI/P/21/13557). Before data collection was initiated in the field, this research study was officially introduced to local administrators (County Commissioner, Deputy County Commissioner, Assistant County Commissioners, Chiefs and Assistant Chiefs) in charge of the study area. No local meetings (barazas) were conducted due to the Ministry of Health guidelines on COVID-19 that were in place. Participants for the livestock blood collection and participatory mapping components were consented separately and individually before signing a form specific to their activity and receiving a copy for their record.
2.2. Blood sample collection, ethics, and data entry
Blood was sampled at one slaughterhouse, Mamboleo Slaughterhouse, which is the largest slaughterhouse in Kisumu City and Western Kenya. For livestock sampling, stakeholders, slaughterhouse managers, and slaughterhouse workers were sensitized to the goals of the project and the stakeholders consented for their animals' blood to be collected after slaughter and tested for RVFV. A team of two field assistants from the slaughterhouse were recruited and trained on blood and data collection methods. This included identifying every second animal in line to be slaughtered as eligible for inclusion (systematic sampling) in the study. Once an animal was identified to be part of the study, the assistant removed the cap on a 15 mL plastic conical tube as the animal was stunned (via captive bolt) and then passed the tube to the slaughterman who was instructed to hold the tube at a 45-degree angle and collect dripping blood immediately after severing the neck of the animal. Labeled blood samples were immediately placed into a cooler box with an ice pack. At the slaughterhouse, cattle were slaughtered in a separate area to small stock (sheep and goats), and all species were systematically sampled to ensure a representative sample was collected from each day's animals.
Each stakeholder or their appointed representative was then located within the slaughterhouse grounds and verbally responded to a brief survey filled out by an assistant on a laminated card matched that matched to the pre-labeled blood tube where the animals' origin, transport means, and purchase market recorded. Study assistants were also trained to count the adult teeth on the animal after the head was removed for later interpretation of age estimate. The first author of this study (a veterinarian) was present for the first week of sampling and had personal oversight of teeth counting and training of field assistants. At the end of the slaughtering session, samples from the day were given a full study identification number from the simple code and data from the laminated card were entered into the Stanford University RedCap database for secure data storage [22].
2.3. Sample size calculation
As this was the first-time epidemiological data for RVFV was recorded for the independent variables of interest in an urban setting, we had little baseline data to guide calculation of sample size and therefore size calculation was based on hypothesis testing rather than precision. For the slaughterhouse blood sampling, our sampling target was calculated to be 291 to be able to detect a 10% difference between groups with 80% power and 95% precision.
2.4. Laboratory analysis
Blood samples were transported in a cooler box to the laboratory within four hours of collection and on arrival, serum was separated by centrifugation, aliquoted, and stored at —80C for later testing. All samples were tested individually for anti-RVFV IgG antibodies to determine if the animal had been exposed to RVFV over its lifetime. The laboratory assay we used was a commercially available competitive Enzyme-Linked Immunosorbent Assay (c-ELISA) kit used to identify anti-RVFV IgG directed against the nucleoprotein (NP). The assay was carried out and interpreted as per the manufacturers protocol (Innovative Diagnostics, Grabels, France) with the optical density (OD) read at 450 nm.
2.5. Participatory mapping
After the blood collection component of the study was completed, we gathered 12 stakeholders from the two main slaughterhouses (Mamboleo and Rabuor) serving the urban area in two separate FGDs. Each group had six male participants and a trained moderator guided them to share information about the origins of their livestock and influences on the transport routes they use. Each FGD was provided with a large poster board and marker pens and encouraged to visually display the marketplaces and the routes they take to transport the animals to reach their specific slaughterhouse. In addition to geographical information represented on the poster, the stakeholders were probed to describe factors that influence their route choices and the processes at each stage of purchase. Meetings were recorded and transcribed verbatim and the posters and transcripts served as the basis to develop an electronic copy of the map. Transcript analysis was carried out by hand where markets, reference points, and the slaughterhouses identified as GPS points and route descriptions were recorded in an excel file. GPS points were identified for the slaughterhouses, markets, and reference points by using Google Earth, online version. All transport routes were mapped according to an open-source shape file on main, secondary, and tertiary roads in Kenya. A thematic analysis of themes to explain the structure of the maps was carried out and notable quotes on key points were matched to the point they were described and overlaid on the map as annotations. All spatial representation of GPS data was carried out using QGIS Version 3.22.3 with imported online open-source shapefiles (https://africaopendata.org).
Once the initial points and routes were identified, the map was printed on a large poster and another meeting with stakeholders was organized to validate the findings. Stakeholders were asked to verify that the markets were represented correctly and instructed to add markets that were missed in the initial mapping process. Corrections and additions were completed before printing out the final version for each slaughterhouse to hang in the respective meeting rooms at their slaughterhouses.
2.6. Data analysis
Descriptive statistics of the slaughterhouse blood samples were computed and the dependent variable (outcome) for analysis was each individual samples' binary anti-RVFV antibody exposure status, which was defined as either positive or negative for IgG antibodies. Independent variables included species, origin location, markets the animal passed through, transport means, total animals arriving with the herd, and the animal's estimated age. All statistical and descriptive analyses were performed in R via RStudio (2022.02.3). Data collected from this study has been deposited in the Stanford Digital Repository and is available for download at https://purl.stanford.edu/cf760nk5627.
Variable effects were initially explored by cross tabulating results and data visualization. Bivariable analysis was carried out to determine the effect of independent variables on the RVFV exposure status (outcome). Thereafter, logistic regression was used to determine the significance of the independent variables on RVFV exposure status (outcome). After bivariable analysis, multivariate generalized linear regression models were built to determine the combined effect of independent variables and define significant predictors of RVFV exposure status. Variables that were initially significant in the bivariable analysis were considered for the multivariable model, and additional plausible variables were added one at a time as the Akaike information criterion (AIC) was monitored to determine if the variable addition improved the fit of the model. When the independent variables added to the model increased the AIC and resulted in no significant predictors, it was removed. To explore effects independent of species, separate models were built for cattle and small stock (sheep and goats).
3. Results
Over 11 sampling days, excluding weekends, this study collected a total of 304 blood samples from 114 cattle, 131 sheep, and 59 goats. Overall, we identified a 9% (28/304) IgG seroprevalence and cattle had an 18% seroprevalence, goats 7% and sheep 3%. Animals waited up to 12 days to be slaughtered, and 75% were slaughtered within three days of arriving (Table 1). The majority (86%, 24/28) of seropositive animals arrived at the slaughterhouse in a lorry and only one seropositive sheep and one goat walked to the slaughterhouse. Additionally, 23% (26/114) of cattle walked to arrive at the slaughterhouse and the walking animal originated primarily from Kisumu County (n = 24). Herd size varied greatly (1−100), and herd size and transport means were associated with larger herd sizes arriving on lorries (p < 0.001).
Table 1.
Descriptive statistics of slaughtered animal's RVFV exposure status.
| Total (n) | Anti-RVFV IgG + (%) | P-value | ||
|---|---|---|---|---|
| Overall | 304 | 28 (9) | ||
| Species | Sheep⁎ | 131 | 4 (3) | |
| Cattle | 114 | 20 (18) | <0.001 | |
| Goats | 59 | 4 (7) | 0.24 | |
| Days waiting for slaughter | 1–3 | 228 | 19 (8.3) | 0.07 |
| 4–6 | 30 | 8 (26.7) | ||
| 6–8 | 17 | 0 | ||
| 9–12 | 1 | 0 | ||
| Herd size received | 1–20 | 197 | 23 (11.7) | 0.08 |
| 21–40 | 31 | 3 (9.6) | ||
| 41–60 | 47 | 0 | ||
| 60+ | 26 | 2 (7.7) | ||
| N/A | 3 | |||
| Purchased from a market | Yes | 301 | 27 (9) | 0.19 |
| No | 3 | 1 (33) | ||
| Transport means | Lorry⁎ | 205 | 24 (12) | |
| Walked | 28 | 1 (4) | 0.22 | |
| Tuk-tuk | 71 | 3 (4) | 0.08 | |
| Estimated age (years) | < 2⁎ | 79 | 6 (8) | |
| 2 | 53 | 3 (6) | 0.66 | |
| 3 | 88 | 7 (8) | 0.93 | |
| 4 | 39 | 4 (10) | 0.62 | |
| 5+ | 36 | 8 (22) | 0.03 | |
| Age N/A | 10 | |||
| Origin county | Homabay⁎ | 43 | 1 (2) | |
| Kericho | 3 | 0 | 0.99 | |
| Kisumu | 73 | 3 (4) | 0.64 | |
| Migori | 50 | 17 (34) | 0.11 | |
| Nakuru | 37 | 2 (5) | 0.51 | |
| Nandi | 16 | 3 (19) | 0.08 | |
| Narok | 4 | 0 | 0.99 | |
| Siaya | 51 | 2 (4) | 0.69 |
P-value computed by logistic regression.
Bold P-values are >0.05 indicating statistical significaance
Reference for categorical variable.
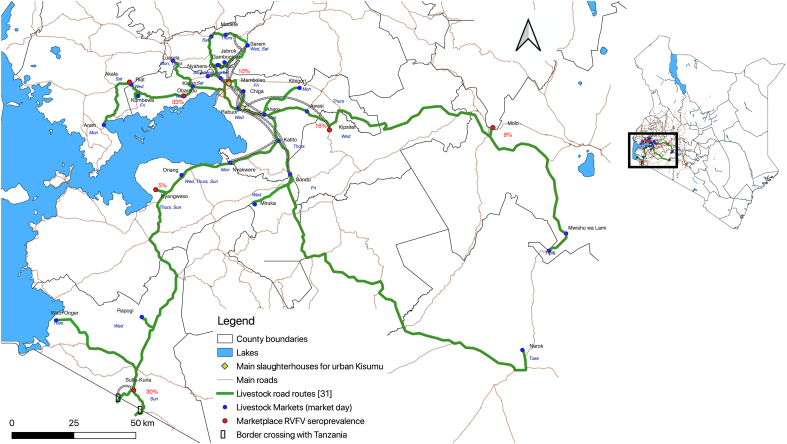
The majority (99%) of animals were purchased at a livestock market and all seropositive animals were purchased from livestock markets except one goat originating from Kisumu that arrived at the slaughterhouse on a tuk-tuk (A small taxi built on a Bajaj motorcycle body). The local urban animal's purchase history was unknown; however, it was estimated to be less than two years old. Study animals were purchased at 23 unique markets across eight counties in Western Kenya . Of those 23 markets, eight markets provided animals to the urban slaughterhouse that had been exposed to RVFV and 60% (17/28) of all positive samples were from animals purchased at the Suba Kuria market in Migori County. Of the 57 total animals that were sourced from Suba Kuria, 91% (52/57) were cattle and 31% of the cattle (16/52) were RVFV seropositive. A summary of county level seroprevalence is shown in Fig. 1, and Migori County had the highest RVFV exposure rate per county.
Fig. 1.
Sampling area and seroprevalence by reported origin County.
Overall, 97% (295/304) of animals had their adult teeth counted and age estimated. When field assistants encountered an unusual dental presentation (missing teeth, worn teeth, atypically shaped tooth, etc.), they sent a photo from their smart phone to the first author of this manuscript for confirmation. The distribution of age was slightly left-skewed as the group <2 years was overrepresented (zero adult teeth). Nonetheless, animals estimated to be greater than five years old were significantly more likely to have lifetime exposure to RVFV (p = 0.03).
There were species differences for nearly every independent variable. Cattle were either transported on a lorry or walked, and small stock (sheep and goats) were the only species to be transported in a tuk-tuk. Cattle and sheep had a larger number of animals received with the herd compared to goats (p < 0.001), and cattle and sheep waited longer than goats to be slaughtered (p < 0.001). Cattle also tended to be older than sheep and goats, with 46% (52/114) of cattle, only 12% (7/59) of goats, and 12% (16/131) of sheep being >4 years old (p < 0.001). For sheep, 32% (42/131) were less than two years old.
3.1. Multi-variable modeling
The final multivariable statistical model selected for this study is summarized in Appendix A.2. The final model shows that species (Cattle, OR = 9.2, p = 0.001) and transport means (Walking, OR = 0.2, p = 0.06) to the slaughterhouse were variables that when considered together, best predicted RVFV seropositivity and walking was protective effect. Multivariable model construction was complicated by species being associated with nearly all other variables. Species type was a major determinant of the processes carried out at the slaughterhouse because the other variables were related to systems driven by business decisions, capacity, and available infrastructure. However, given that cattle species was a significant predictor with a low AIC in the bivariable modeling, species was chosen as the base variable for all models and the effects of adding additional variables were tracked using the AIC and determining if additional variables were also significant. A full summary of significant variable tracking and AIC is provided as Appendix A.1. Briefly, age and species were associated, and when these variables were combined in the same model, the AIC was higher, and the species (cattle) remained the only significant variable (p = 0.001). Older age was significant only when species was removed, and when age was combined with number of days waiting for slaughter, a non-correlated variable. A significant effect of origin county was only observed when county was combined with species; however, the AIC was higher in this model compared to the selected model (species and transport means).
Models were built separately for cattle and small stock (sheep and goats) and none of the independent variables were associated with RVFV seropositivity other than the number of animals received with the herd predicting cattle seropositivity approaching significance (p = 0.06) (Appendix A.1). Otherwise, the species-specific multivariable models did not yield statistically significant results. Interestingly, age and origin county (individually and combined) were not significant in either individual species model which further reinforced the effect of species on our outcome of interest, RVFV exposure status.
3.2. Qualitative and spatial component results
The main stakeholders from each of the two slaughterhouses that serve the urban area were recruited to the participatory mapping component of our study. Participants included 12 men, six from each main slaughterhouse, that were overall motivated to provide information about the livestock markets they visited and understood the importance of tracking this for disease management. They requested to display the market day on the poster alongside the name as this was the primary determinant of where to go purchase animals on a given day. In addition to providing location and route landmarks, stakeholders were probed to report on their operations and what influenced livestock selection and transport decisions. A summary of all marketplaces and routes mentioned during the participatory mapping exercise that serve the urban center are summarized in Fig. 2. Thereafter, specific portions of the map of Western Kenya have been zoomed in to demonstrate the key quotations as qualitative data matching the findings below linked to specific points and marketplaces (Fig. 3, Fig. 4, Fig. 5).
Fig. 2.
Marketplaces serving the urban Kisumu slaughterhouses and movement routes.
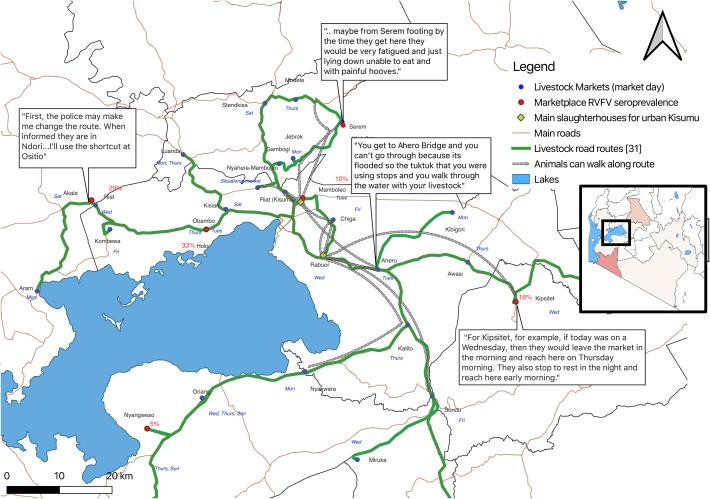
Fig. 3.
Border crossing with Tanzania and Subakuria market dynamics.
Fig. 4.
Local market suppliers and storage locations in urban homesteads.
Fig. 5.
Walking routes and route deviations.
3.3. Market processes and selection influences
All mapping participants affirmed that livestock markets are indeed the key places for purchasing animals and these markets are vitally important for business because they can provide an adequate number of healthy animals. Participants reported that although they moved livestock long distances from the market to the slaughterhouse, each of the markets were primarily supplied by local animals residing in the surrounding areas. This contrasts with one particularly large market, located near to the Kenyan border with Tanzania. Suba Kuria market is in Migori County and is a preferred market as it is said to always supply strong and healthy cattle. Animals can travel long distances and arrive at this market from Tanzania to be sold for a higher price in Kenya. Stakeholders reported that animals cross the border at two points, one official and one unofficial. At the unofficial crossing, the animals alight their transport lorry when they arrive at the border and then proceed to walk the remainder of the distance to the marketplace.
Additionally, animals do not sleep overnight at the marketplaces and instead arrive only on the morning of market day. If animals were unsold after the end of the market day, they either returned home with their owner or were moved to the nearest market on a subsequent market day. It was noted that because the markets leading into Kisumu are not held on consecutive days, it was indeed possible for an animal to have multiple movements further away from the urban center before ultimately being slaughtered at an urban Kisumu slaughterhouse. There were no official movement permits to track these between market movements other than a permit that was issued at the point of sale to bring the animal across county borders to slaughter.
When stakeholders selected an animal for purchase, they considered the transport means because if the animal was supposed to walk to the urban slaughterhouse, it needed healthy and durable hooves to withstand transport. Additional factors that influenced the selection of each individual animal was the animals' general health, the price, color of the animal, the seller's integrity. The color of the animal was said to be important if the cattle were intended as a payment for marriage as it was taboo to offer black or multi-colored cattle in Luo (most populous tribe in Kisumu) culture. The animal's health was reported to be difficult to ascertain at the marketplaces and sometimes, and seemingly healthy animals were purchased, but did not survive the journey to the slaughterhouse. Stakeholders reported that previously a veterinarian was present at the large Suba Kuria marketplace to guide their purchasing strategy, but this was no longer the case. However, another participant added that if you were going to keep the animal in your personal herd, a veterinarian would be able to inspect it for you, but for animals intended for slaughter, they primarily focused on the animal's body condition.
Participants reported that few animals were slaughtered from the surrounding urban area as there was simply not enough animals to meet the demand of urban butchers. Interestingly, at Rabuor slaughterhouse, there was not a designated space for animals to await slaughter and the stakeholders had to make private arrangements with nearby homesteads to care for their animals. (Fig. 5). Here, foreign livestock could reside at the urban household for up to two weeks and mix with local urban livestock while awaiting slaughter. At both slaughterhouses, participants noted that it was important to let the animals rest before slaughtering, and if the journey was long, this could take several days. This confirmed our quantitative date showing that indeed, cattle can be kept in holding pens for extended periods of time before slaughter. A participant also added that health of the animal sometimes dictates if it is slaughtered quickly or kept in holding longer.
“Any cattle that has been stepped on and unable to stand can never be slaughtered immediately. They must wait for 2-3 days in the sun, so that blood may run swiftly in their bodies before slaughtering, but the ones with painful hooves are slaughtered because they have not eaten and might die.”
As Rabuor slaughterhouse was located along the main road entering Kisumu from the South, their primary markets were in this southward direction, and they did not visit markets on the North lakeshore (Fig. 5). Aside from this, there was little difference between sourcing locations for the two slaughterhouses.
3.4. Transport means, route influences, and deviations
All markets were located adjacent to main roads and all transport routes were along main and secondary roads, although road quality from market to Kisumu varied greatly. Transport means included lorry, tuk-tuk, and pickup trucks. Animals that walked to the slaughterhouse also traveled along the roadside as this was said to be the most straightforward pathway. Animals and their herders sometimes had to move at night if they had a long distance to cover. Walking animals were not allowed to drink water while in transport as this apparently caused them to “swell,” and often they would die. However, animals were allowed to graze if the journey spanned several days, and they stopped to rest near to the roadside at night.
Influences on the transport means included the species (small vs large stock), total number purchased, cost of the transport, and lorry availability. The stakeholders said they would only be able to justify the lorry fee if they were able to purchase many animals at once. Deviations from the planned route included unexpected flooding, police checkpoints, and livestock health decline. If the walking animal had sore hooves and began to have difficulty walking, the stakeholder would attempt to organize transport for animal, but in case where they were unable to find alternative means, they identified a slaughterhouse along the route to slaughter the animal and proceeded to transport the meat on a motorbike the remaining distance to Kisumu. Otherwise, the stakeholders did not sell animals they had purchased along the route and instead were motivated to reach the urban slaughterhouses in Kisumu with live animals.
The highest demand for meat in Kisumu was determined to be in December, and this shift in demand opened a seasonal livestock market, Wath-Onger, in Migori County. An additional circumstance for a situational livestock market to “pop up” was when the main livestock market was closed for various reasons, including “an outbreak.” Nonetheless, even when reporting challenges, stakeholders expressed pride in their business knowing they are providing a means for urban populations to consume highly demanded red meat.
4. Discussion
In this study, we have demonstrated the feasibility of a new sampling framework for wide-spread RVFV surveillance in Western Kenya by collecting blood from urban slaughtered livestock. Our data have demonstrated regional RVFV risk and provided additional evidence against the previous classification of Western Kenya as a low-risk area for RVFV transmission [23]. Interpretation of RVFV risk zones must be viewed as highly dynamic as RVFV disease ecology is particularly vulnerable to the effects of climate change, population growth, urbanization, and land use change [6,24,25]. Our spatial data on livestock markets and routes have provided context for interpreting RVFV exposure risk and could guide future surveillance efforts when a single case is identified to obtain more primary data for urban risk assessment. The livestock movement routes displayed here could also be used for management of other livestock diseases, such as Foot and Mouth disease, in Western Kenya.
Slaughterhouses have an increased human burden of zoonotic diseases, including RVF, and are key locations for disease exchange from animals to humans [10,26]. Slaughterhouses have also provided a basis for other routine surveillance programs for priority diseases in Kenya by requiring a veterinary professional to visually screen animal carcasses on post-mortem examinations for classic lesions. However, monitoring this data over time is complicated by inconsistent and inaccessible paper record keeping. A study across several slaughterhouses in Western Kenya established that an electronic record keeping system for post-mortem findings was feasible and capturing animal origin was a primary challenge in their study [27]. In our study, we were also rarely able to capture the animal's birth village, yet had a 100% success rate in documenting the market the animal was purchased from. Qualitative data from stakeholders enriched interpretation of this data by explaining that most rural markets were supplied locally from surrounding areas and in some cases, the stakeholders knew the villages that supplied certain markets. Thus, our base map of marketplaces could be expanded with additional participatory techniques at the named marketplaces using similar methods to identify all supplying villages and routes.
We identified species, age, and means of transport as factors that can predict RVFV seropositivity at the urban slaughterhouse and identified Migori County, where the Suba Kuria market is located, to have the highest county-level seroprevalence. The majority (80%) of seropositive cattle in our study were purchased here; however, in the species-specific logistic regression models, neither county nor marketplace was significantly associated with RVFV seropositivity. This effect may be due to the small and unequal sample sizes from different counties and future studies should increase their sample size to account for numerous strata and confounding variables. The Suba Kuria market was a preferred marketplace by stakeholders, and the animals purchased there that were intended for slaughter did not have the same inspection process as those purchased as local herd additions. All animals arriving at the urban slaughterhouse from Suba Kuria market traveled in a lorry to reach the slaughterhouse, and to justify the cost, stakeholders purchased many animals at once for transport. As slaughtering day of choice is primarily directed by orders received from butchers, these animals waited in holding pens for up to one week to be slaughtered, with cattle waiting longer than sheep and goats. The incubation period for RVFV in adult livestock is between two and six days and therefore, if an animal was infected with RVFV before arrival, it may reach peak viremia while in the holding pen [1]. Overall, our initial results indicate that cattle have the highest seroprevalence rate of RVFV which is confounded with age, but with their longer waiting periods, cattle would most likely have the highest risk to initiate an urban transmission cycle in vectors. Given our data, when diagnostic resources are limited, focus for surveillance would be best directed to cattle from high-risk markets, such as the Suba Kuria market. However, another study using within host mathematical modeling methods and viral titer data found that sheep had a higher transmission potential to vectors [28], so perhaps this recommendation to only sample cattle would be insufficient in midst of a large national outbreak.
The qualitative component of this study contributes to a greater understanding of urban RVFV introduction and transmission risks between species and from imported animals to local urban livestock. The livestock transport period from market to slaughter was said to physically exhaust the animals no matter their transport means, and they typically arrive at the slaughterhouse lethargic. Lethargy is a major non-specific sign of illness for RVFV infection in adult cattle. Attributing lethargy to the transport period could delay recognition of lethargy from disease which would result in them spending longer in the holding pen and further increase risk of infecting urban mosquitos. Alternatively, lethargy could be noted as a sign of illness and the animal might be slaughtered promptly and risk direct transmission of RVFV to slaughterhouse workers. Ideally, suspect animals would be screened prior to movement and slaughterhouse workers and stakeholders would use personal protective equipment (PPE) when handling these animals. Stakeholders at the Rabuor slaughterhouse shared that imported animals wait at urban homesteads to be slaughtered when they do not have access to a specific holding pen at the slaughterhouse. As RVFV does not transmit horizontally animal to animal even when animals are immunocompromised [29], risk of transmission between species could be greater in the urban environment as mosquito vectors are more abundant [14]. A better understanding of mosquito ecology at key transmission points, such as slaughterhouses, is required to qualify this risk and guide vector control measures. The lack of access to diagnostic testing for RVFV suspected animals is a major concern and widespread availability of rapid diagnostic assays for RVFV, with appropriate access to gold standard confirmatory tests, would prove especially useful in a slaughterhouse setting. Yet, if an animal tests positive for RVFV at the slaughterhouse, it may not necessarily have been infected at its origin, particularly if transport was over several days and the animal was moved at night. Market towns by the roadside are key areas of human, animal, and vector congregation which results in a wider, more interconnected interface for RVFV transmission in the urban environment compared to rural homesteads [30]. It is notable that one of our exposed animals was recorded to be a local urban goat from Kisumu City. This animal's full history was unknown, so we cannot infer that it was infected in the urban setting. However, detection of RVFV in an animal that has been sourced from a rural primary marketplace could indicate RVFV circulation in the surrounding community, and such finding should trigger additional localized surveillance, informing veterinary services, and alerting human health centers of the potential to receive human RVFV cases.
We did not work directly with meat inspectors, and a limitation of this study is not being able to link our laboratory data to any post-mortem findings. RVFV post-mortem lesions have been primarily described in young animals and abortion tissues as young animals are more affected and available for necropsy, displaying characteristic diffuse necrotizing hepatitis with multifocal necrosis [31]. Given that adult cattle primarily have inapparent infection (no detectable clinical signs), they are rarely tested for RVFV and therefore, post-mortem lesions have not been extensively described in this population, particularly during the interepidemic period. Integration of post-mortem data with our sampling framework and an electronic record keeping system, may improve classification of RVFV associated lesions in asymptomatically infected or recently exposed adult animals in endemic regions. Relevant to lesion recognition and comparing risk over time, our study was able to estimate the age of animals by having field assistants count the number of adult teeth after the head was removed. As with most RVFV livestock seroprevalence studies, we showed higher exposure rates in animals greater than five years old except for the group less than two years old. This effect was explored retrospectively with the field team, and our hypothesis was that assistants sometimes recorded “zero adult teeth” to include the very old animals that had lost all teeth, and these animals were then classified in the less than two years' age group. This nuance should be emphasized in future training programs when lay individuals are instructed to estimate age by dentition. We also only tested blood samples for anti-RVFV IgG antibodies which limits our ability to describe recent RVFV circulation in Western Kenya. In future sampling efforts, testing for RVFV antigen and IgM antibodies could indicate areas where recent transmission has occurred and a more up-to-date classification of risk areas prone to unrecognized smaller outbreaks. An additional limitation of this study is the potential bias in only collecting movement data from businessmen and not confirming our findings with use of GPS trackers, and future approaches to assess livestock movements may take advantage of multimodal approaches. However, we emphasize the need to engage local individuals whose livelihoods rely on livestock movements regardless of data collection methods.
This surveillance framework could be rapidly locally implemented by trained assistants at the slaughterhouse in response to detection of a single RVFV case or early warning alert for high regional risk. We were able to reliably collect total animals received with the herd, waiting times for slaughter, estimated the age, and origin locations by proxy for the marketplace the animal was purchased from. We anticipate this study to have high reproducibility at other slaughterhouses in the region because the sampling approach is integrated into the typical flow of process and business. Improvement of RVFV surveillance in livestock remains a priority as it has direct impacts on public health. Identification of more acute endemic cases over time is vital to a better understanding of RVFV epidemiology and quantifying future endemic risk of RVFV transmission at the humans-livestock-vector interface in urban areas.
5. Conclusion
We conclude that sampling of blood from livestock directly after their slaughter is a practical approach to implement active livestock surveillance during high-risk times and may be more cost-effective approach for active RVFV surveillance than sampling live animals. Key points for the success of this framework include extensive sensitization of the slaughterhouse stakeholders and training of local field assistants from the slaughterhouse to collect samples and epidemiological data as they are already integrated into the normal slaughterhouse procedures and trusted by stakeholders.
Our livestock movement maps created in collaboration with key stakeholders have provided a means to display the lived experiences of moving livestock and these maps likely have more relevance than if they were solely created by select dominant figures in livestock systems. Additionally, through this participatory process, stakeholders have been sensitized to the rationale of movement and disease spread. Movement restrictions that are based on specific routes rather than county-to-county permit administration would be expected to have a greater impact on disease spread while sparing more livelihoods relying on livestock. Indeed disease control measures within livestock systems that consider business pressures experienced by key stakeholders are also likely to have better compliance and can contribute to building local resilience for disease management. With the projected demand for livestock in urban areas to continue rising, risk of diseases such as RVFV is also likely to increase. Integrated surveillance systems such as the one presented here will be a key in recognizing disease introductions and monitoring dynamic risk in real-time.
Funding sources
This study was funded by Stanford University's Center for Innovation in Global Health (CIGH) Seed Grant Program (Gerken, LaBeaud, and Seetah: Co-primary investigators). The project received additional support from the Robert E. Shope International Fellowship in Infectious Diseases (Gerken, 2021 award recipient) from the American Society for Tropical Medicine and Hygiene (ASTMH). The funders had no role in the design of this study or in the decision to publish.
CRediT authorship contribution statement
Keli Nicole Gerken: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft. Bryson Alberto Ndenga: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. Kevin Omondi Owuor: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. Christabel Achieng Winter: Data curation, Investigation, Visualization, Writing – review & editing. Krish Seetah: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – review & editing. Angelle Desiree LaBeaud: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing.
Declaration of Competing Interest
All authors included on of this manuscript declare that they have no conflicts of interest.
Acknowledgments
We thank the local administrators in the study area, slaughterhouse workers and stakeholders at the Mamboleo and Rabuor slaughterhouses in Kisumu. We specifically thank Mr. Victor Otieno and Mr. Jacob Sule for their hard work during data collection, Mrs. Beatrice Obadha, Mr. Jackonea Sule, and Mr. James Nyabora for their dedicated support for this research study. We also acknowledge Ms Stella Orwa's contributions in the focus group discussions and transcribing qualitative data. This paper is published with the permission from the Director General Kenya Medical Research Institute.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2022.100457.
Appendix A. Supplementary data
Table of multivariable model outputs and tracking of included independent variables
Final multivariable model selected
Data availability
Data will be made available on request.
References
- 1.WHO Rift Valley fever Fact Sheet [Internet] 2018. https://www.who.int/news-room/fact-sheets/detail/rift-valley-fever [cited 2021 Sep 16]. Available from:
- 2.Muga G.O., Onyango-Ouma W., Sang R., Affognon H. Review article: sociocultural and economic dimensions of Rift Valley fever. Am. J. Trop. Med. Hyg. 2015;92(4):730–738. doi: 10.4269/ajtmh.14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faburay B., LaBeaud A.D., McVey D.S., Wilson W.C., Richt J.A. Current status of rift valley fever vaccine development. Vaccines. 2017;5(3):1–20. doi: 10.3390/vaccines5030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bird B.H., Nichol S.T. Breaking the chain: Rift Valley fever virus control via livestock vaccination. Curr. Opin. Virol. 2012;2(3):315–323. doi: 10.1016/j.coviro.2012.02.017. Available from: [DOI] [PubMed] [Google Scholar]
- 5.Gerken K.N., LaBeaud A.D., Mandi H., L’Azou Jackson M., Breugelmans J.G., King C.H. Paving the way for human vaccination against Rift Valley fever virus: A systematic literature review of RVFV epidemiology from 1999 to 2021. PLOS Neglected Tropical Diseases. 2022;16(1):e0009852. doi: 10.1371/journal.pntd.0009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerken K.N., LaBeaud A.D., Mandi H., L’Azou Jackson M., Breugelmans J.G., King C.H. Paving the way for human vaccination against Rift Valley fever virus: A systematic literature review of RVFV epidemiology from 1999 to 2021. PLoS Negl. Trop. Dis. 2022;16:e0009852. doi: 10.1371/journal.pntd.0009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(IFAD) IF for AD . 2013. Smallholders, food security, and the environment. [Google Scholar]
- 8.Gerken K.N., Maluni J., Mutuku F., Ndenga B., Ichura C., Mwashee L., et al. “We just considered it [Rift Valley Fever virus] to be over there”: A qualitative study exploring urban perspectives for disease introduction. medRxiv. 2022 http://medrxiv.org/content/early/2022/05/07/2022.05.05.22274700.abstract 2022.05.05.22274700. Available from: In preparation. [Google Scholar]
- 9.Kenyalaw. Kenya Law Rep; 2012. Meat Control Act. [Google Scholar]
- 10.Cook E.A.J., De Glanville W.A., Thomas L.F., Kariuki S., Bronsvoort B.M., Fèvre E.M. Working conditions and public health risks in slaughterhouses in western Kenya. BMC Public Health. 2017;17(1):1–12. doi: 10.1186/s12889-016-3923-y. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glyn Davies F., Martin Vincent. FAO Animal; Rome: 2003. Recognizing Rift Valley Fever. [PubMed] [Google Scholar]
- 12.Mutua E., De Haan N., Tumusiime D., Jost C., Bett B. A qualitative study on gendered barriers to livestock vaccine uptake in Kenya and Uganda and their implications on rift valley fever control. Vaccines. 2019;7(3):1–22. doi: 10.3390/vaccines7030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Bergh C., Venter E.H., Swanepoel R., Thompson P.N. High seroconversion rate to rift valley fever virus in cattle and goats in far northern Kwazulu-Natal, South Africa, in the absence of reported outbreaks. PLoS Negl. Trop. Dis. 2019;13(5):1–19. doi: 10.1371/journal.pntd.0007296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ndenga B.A., Mutuku F.M., Ngugi H.N., Mbakaya J.O., Aswani P., Musunzaji P.S., et al. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS One. 2017;12(12):1–14. doi: 10.1371/journal.pone.0189971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Rezende I.M., Sacchetto L., Munhoz E., Augusto Alves P., de Melo Campos, Iani F., Ribeiro Adelino M., et al. Persistence of Yellow fever virus outside the Amazon Basin, causing epidemics in Southeast Brazil, from 2016 to 2018 Izabela Maurício de Rezende 1, Lívia Sacchetto 1. Erica Munhoz de Mello. 2018:1–12. doi: 10.1371/journal.pntd.0006538. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Glanville W.A., Allan K.J., Nyarobi J.M., Thomas K.M., Lankester F., Kibona T.J., et al. An outbreak of Rift Valley fever among peri-urban dairy cattle in northern Tanzania. Trans. R. Soc. Trop. Med. Hyg. 2022;1–9 doi: 10.1093/trstmh/trac076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaBeaud A.D., Pfeil S., Muiruri S., Dahir S., Sutherland L.J., Traylor Z., et al. Factors associated with severe human Rift Valley fever in Sangailu, Garissa County, Kenya. PLoS Negl. Trop. Dis. 2015;9(3):1–14. doi: 10.1371/journal.pntd.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Floyd J.R., Ruktanonchai N.W., Wardrop N., Tatem A.J., Ogola J., Fèvre E.M. Exploring fine-scale human and livestock movement in western Kenya. One Heal. 2019;7(2018):100081. doi: 10.1016/j.onehlt.2019.100081. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jupp D., Ibn Ali S., Barahona C. Sida Studies in Evaluation. vol. 1. 2010. Measuring empowerment? Ask them. [Internet] 1–108 p. Available from: [Google Scholar]
- 20.County Government of Kisumu County Urban Institutional Development Strategy - Kisumu City. 2018:1–21. [Google Scholar]
- 21.International Society for Infectious Diseases (ISID) ProMed RIFT VALLEY FEVER - EASTERN AFRICA: LIVESTOCK, HUMAN, ALERT, FAO [Internet] 2022. https://promedmail.org/promed-post/?id=20220224.8701623 Available from:
- 22.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munyua P.M., Murithi R.M., Ithondeka P., Hightower A., Thumbi S.M., Anyangu S.A., et al. Predictive factors and risk mapping for Rift Valley fever epidemics in Kenya. PLoS One. 2016;11(1):1–13. doi: 10.1371/journal.pone.0144570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguirre A.A. Changing patterns of emerging zoonotic diseases in wildlife, domestic animals, and humans linked to biodiversity loss and globalization. ILAR J. 2017;58(3):315–318. doi: 10.1093/ilar/ilx035. [DOI] [PubMed] [Google Scholar]
- 25.Mweya C.N., Mboera L.E.G., Kimera S.I. Climate influence on emerging risk areas for rift valley fever epidemics in Tanzania. Am. J. Trop. Med. Hyg. 2017;97(1):109–114. doi: 10.4269/ajtmh.16-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook E.A.J., Grossi-Soyster E.N., de Glanville W.A., Thomas L.F., Kariuki S., Bronsvoort B.M., et al. The sero-epidemiology of Rift Valley fever in people in the Lake Victoria Basin of western Kenya. PLoS Negl. Trop. Dis. 2017;11(7):1–18. doi: 10.1371/journal.pntd.0005731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falzon L.C., Ogola J.G., Odinga C.O., Naboyshchikov L., Fèvre E.M., Berezowski J. Electronic data collection to enhance disease surveillance at the slaughterhouse in a smallholder production system. Sci. Rep. 2021;11(1):1–13. doi: 10.1038/s41598-021-98495-7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cecilia H., Vriens R., Wichgers Schreur P.J., de Wit M.M., Métras R., Ezanno P., et al. Heterogeneity of Rift Valley fever virus transmission potential across livestock hosts, quantified through a model-based analysis of host viral load and vector infection. PLoS Comput. Biol. 2022;18(7):1–19. doi: 10.1371/journal.pcbi.1010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wichgers Schreur P.J., Van Keulen L., Kant J., Oreshkova N., Moormann R.J.M., Kortekaas J. Co-housing of Rift Valley fever virus infected lambs with immunocompetent or immunosuppressed lambs does not result in virus transmission. Front. Microbiol. 2016;7(MAR):1–9. doi: 10.3389/fmicb.2016.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassell J.M., Begon M., Ward M.J., Fèvre E.M. Urbanization and disease emergence: dynamics at the wildlife–livestock–human Interface. Trends Ecol. Evol. 2017;32(1):55–67. doi: 10.1016/j.tree.2016.09.012. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odendaal L., Davis A.S., Fosgate G.T., Clift S.J. Lesions and cellular tropism of natural Rift Valley fever virus infection in young lambs. Vet. Pathol. 2020;57(1):66–81. doi: 10.1177/0300985819882633. [DOI] [PubMed] [Google Scholar]
- 32.Gerken KN, Mutuku FM, Ndenga BA, Agola GA, Migliore E, et al. Urban risk factors for human Rift Valley fever virus exposure in Kenya. PLOS Global Public Health. 2022;2(7) doi: 10.1371/journal.pgph.0000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table of multivariable model outputs and tracking of included independent variables
Final multivariable model selected
Data Availability Statement
Data will be made available on request.