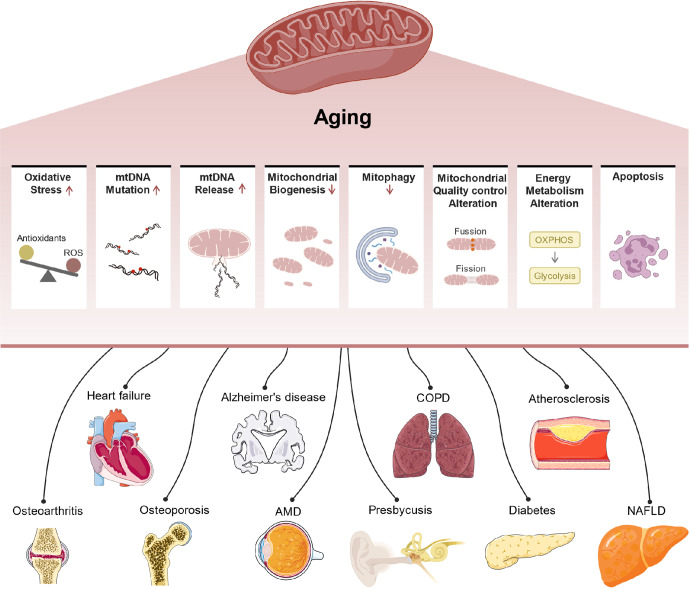
Fig. 5.
Mitochondrial dysfunction contributes to diverse aging-related diseases. With aging, an increase in ROS production in mitochondria leads to oxidative stress, causing oxidative damage to DNA (especially mtDNA), lipids, and proteins. An increased mtDNA mutation rate causes increased frequencies of errors or mutations in mtDNA-encoded enzyme subunits, resulting in impaired OXPHOS. mtDNA is released into the cytoplasm or outside the cell and participates in SASP secretion by activating cGAS-STING pathways. Decreased mitophagy mediated by the PINK1/parkin ubiquitin pathway results in impaired clearance of damaged mitochondria. Reduced mitochondrial biogenesis mediated by PGC1 and NRF decreases the number of newborn mitochondria. During aging, mitochondria show altered quality control changes, Drp1/FIS1-mediated mitochondrial fission decreases, and MFN/OPA-mediated mitochondrial fusion increases, affecting mitochondrial shape and function. The mitophagy defects and mitochondrial dysfunction trigger Aβ and tau accumulation, leading to synaptic dysfunction and cognitive deficits during AD development. The metabolic transition from OXPHOS to glycolysis leads to altered metabolite generation. Mitochondrial pathway-mediated apoptosis is an important form of cell death. Mitochondrial dysfunction contributes to AD, HF, diabetes, OP, OA, presbycusis, NAFLD, COPD, AMD, and atherosclerosis by inducing oxidative stress, inflammation, apoptosis, and metabolic alterations. (Fig. 5 includes modified templates from Servier Medical Art (http://www.servier.com), licensed under a Creative Commons Attribution 3.0 Unported License.)