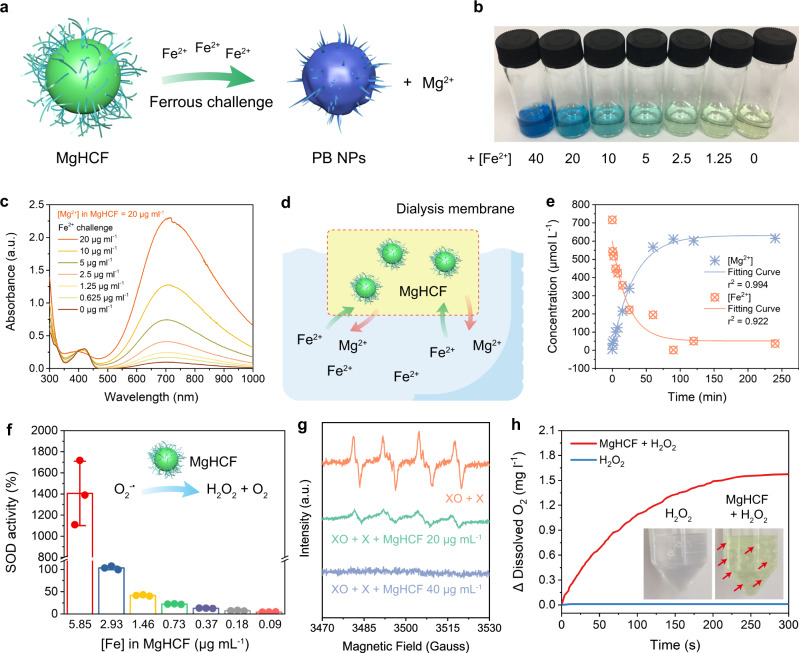
Fig. 3. Ferrous capturing and antioxidation performance of MgHCF NCs.
a Schematic illustration of the ferrous binding activity of MgHCF as a Prussian blue analogue by recovering to the pristine Prussian blue nanoparticles. b Digital photograph of the aqueous solutions containing MgHCF NCs with the additions of the ferrous ions at varying concentrations. c Corresponding UV–vis spectra of respective samples in (b). d Schematic illustration of the dialysis-membrane separation-regulated cation exchange for MgHCF NCs. e Time-dependent elemental concentration profiles for Mg2+ and Fe2+ in the solution during the dialysis-membrane separation-regulated cation exchange for MgHCF NCs. f Superoxide radical dismutation activities of MgHCF at varied [Fe]. n = 3, Data are presented as mean ± s.d. g ESR spectra of MgHCF in eliminating the superoxide anions generated in xanthine oxidase + xanthine system in the presence of BMPO as the radical trapper. h Variation of the dissolved oxygen levels in a H2O2 (2 mM) solution in the presence or absence of MgHCF. The inset shows the digital photograph of samples containing H2O2 or MgHCF + H2O2. Oxygen bubbles are indicated by red arrows.