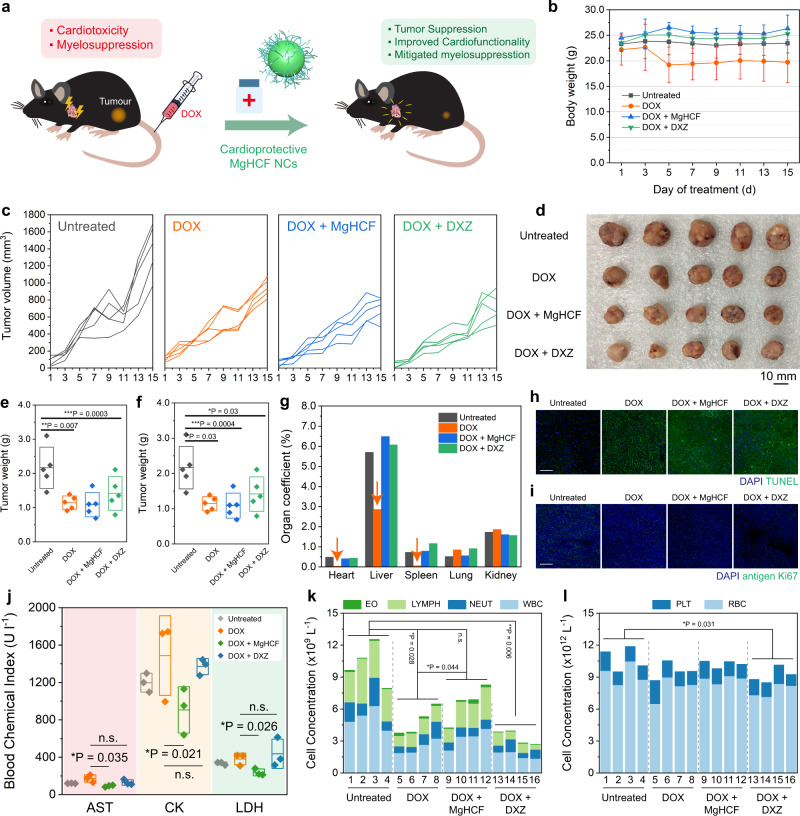
Fig. 8. In vivo anti-tumour performances and side effect evaluation.
a Schematic illustration of the in vivo anti-tumour experiment. b Body weight profiles of mice in different groups during the in vivo anti-tumour investigation. n = 5. Data are presented as mean ± s.d. c Xenograft development curves for each mouse from different groups in the therapeutic evaluation period. d Digital photograph of the dissected tumour xenografts from each mouse of different groups. e and f Tumour weights (e) and tumour volumes (f) of the xenografts dissected from each mouse of different groups. n = 5. Data are presented as they are and mean ± s.d. Statistical significance (to the control group) is assessed by Student t’s two-tailed test. *P < 0.05, **P < 0.01 and ***P < 0.001. g Organ coefficient of mice from different groups, calculated as the ratio of the organ weight to body weight. h and i TUNEL (h) and antigen-Ki67 (i) immunofluorescence staining images of the ultrathin tumour sections dissected from mice of different groups. j Plasma AST, CK and LDH levels of each mouse from different groups. n = 3. Data are presented as they are and mean ± s.d. Statistical significance is assessed by Student t’s two-tailed test. *P < 0.05. k Cell populations of EO, LYMPH, NEUT and WBC were assayed for each mouse from different groups. Data are presented as they are. Statistical significance is assessed by Student t’s two-tailed test. *P < 0.05, **P < 0.01 and n.s. for non-significant. l Cell populations of PLT and WBC were assayed for each mouse from different groups. Data are presented as they are. Statistical significance is assessed by Student t’s two-tailed test. *P < 0.05.