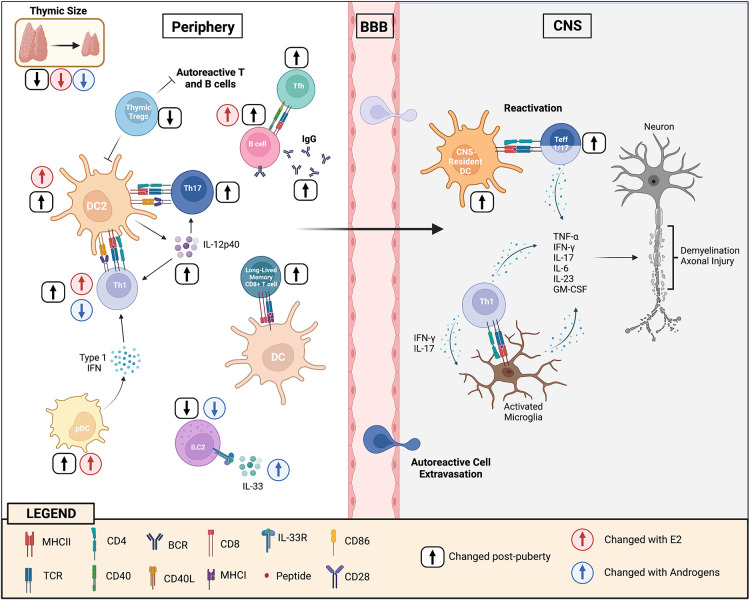
Figure 2.
Puberty augments cellular and humoral immunity to enhance CNS autoimmunity. During puberty, DC2 cells become more mature and efficient at presenting antigen to T helper cells resulting in enhanced Th1 and Th17 immunity. This occurs, in part, through increases in the expression of co-stimulatory molecules, but also IL-12p40 (and IL-12 and IL-23) production by these cells. Adoptive transfer EAE studies in mice of pre- and post-pubertal ages have provided evidence that post-pubertal DCs in the CNS may have a higher potential to re-activate Th1/Th17 cells that have reached the CNS site, resulting in increased myelin tissue damage. Increases in E2 with puberty induce higher levels of type I interferon by pDCs which may further enhance Th1 responses. There is evidence that Th1 cells develop more in a female during the post-pubertal state because of the promoting effects of E2 and the inhibiting effects of androgens. T follicular cell responses also develop more efficiently post-puberty which helps promote B cell responses in the germinal center, resulting in higher IgG production. E2 has an effect in increasing the threshold for BCR activation, allowing potential autoreactive B cells to escape deletion in the periphery. The thymus undergoes dramatic changes with puberty resulting in reduced generation of naïve T cells, in particularly Tregs. This loss in thymic output of T cells may trigger the homeostatic proliferation of perinatal T cells that have a higher TCR responsiveness to self-antigen. With maturation, the BCR and TCR responsiveness to self-antigens becomes diminished, favoring the formation of long-lived memory B cell and CD8+ T cell memory cells instead of short-lived effectors. In addition, ILC2 cell numbers decline post-puberty, in part due to suppressive effects of androgens in the bone marrow. Post-pubertal male ILC2s are also more responsive to IL-33 stimulation. Androgens induce ILC2 cells to accumulate in the lymph nodes of male mice during EAE, limiting disease development in this sex through promotion of the Th2 response.