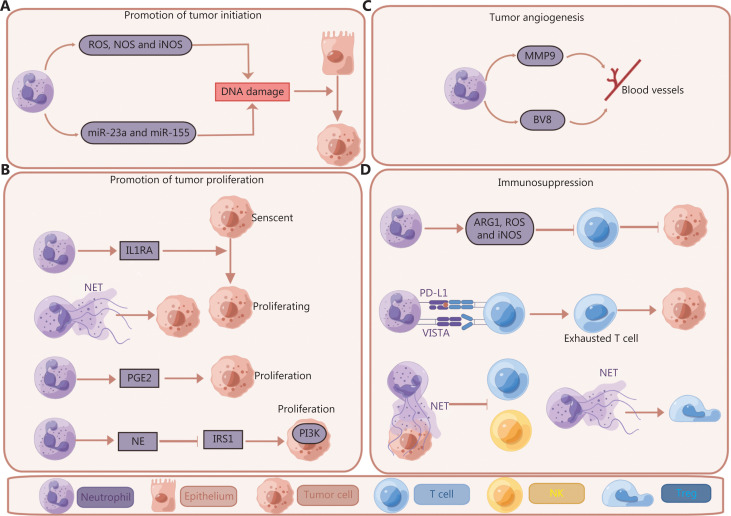
Figure 2.
Neutrophils have protumor properties including causing DNA damage, and promoting tumor proliferation, angiogenesis, and immunosuppression. (A) Neutrophils cause DNA instability through genotoxic DNA substances including ROS, NOS, iNOS, and microRNAs such as miR-23a and miR-155, thus leading to tumor initiation and progression in multiple models (see text). (B) In a PTEN null prostate tumor model, neutrophils promote the proliferation of cancer cells by counteracting senescence with IL1RA production; anaplastic thyroid cancer conditioned medium induces TANs to release NETs in a mitochondrial DNA dependent manner, thereby promoting tumor proliferation. In a RAS-derived neoplasia zebrafish model, neutrophils produce PGE2, thus promoting proliferation of the pre-neoplastic cells in wounded tail fins. In a RAS-induced lung cancer model, neutrophil NE directly induces tumor cell proliferation by infiltrating into the endosomal compartment, degrading IRS-1, and activating PI3K signaling within tumor cells. (C) Neutrophils facilitate tumor angiogenesis via releasing MMP9 and BV8 in a transgenic mouse model with pancreatic islet cell carcinogenesis. (D) Immunosuppressive functions of neutrophils. Neutrophils produce Arg1, ROS, and iNOS, thus impairing the T cell-mediated anti-tumor response. In human hepatocellular carcinomas and gastric cancers, PD-L1+ neutrophils hinder the proliferation and activation of T cells, thus leading to the proliferation and progression of cancer cells. In a melanoma mouse model, VISTA expressed on neutrophils negatively regulates T cell-mediated antitumor immunity; NETs released in the TME form a protective layer on tumor cells and shield them from the cytotoxic activity of CD8+ T cells and NK cells. In a non-alcoholic steatohepatitis-hepatocellular carcinoma (NASH-HCC) model, tumor-induced NETs positively correlate with promotion of Treg differentiation in cancer by metabolic reprogramming of naïve CD4+ T-cells, thereby bolstering hepatocarcinogenesis.