Abstract
For the past few years, platform trials have experienced a significant increase, recently amplified by the COVID-19 pandemic. The implementation of a platform trial is particularly useful in certain pathologies, particularly when there is a significant number of drug candidates to be assessed, a rapid evolution of the standard of care or in situations of urgent need for evaluation, during which the pooling of protocols and infrastructure optimizes the number of patients to be enrolled, the costs, and the deadlines for carrying out the investigation. However, the specificity of platform trials raises methodological, ethical, and regulatory issues, which have been the subject of the round table and which are presented in this article. The round table was also an opportunity to discuss the complexity of sponsorship and data management related to the multiplicity of partners, funding, and governance of these trials, and the level of acceptability of their findings by the competent authorities.
Keywords: Platform trial, Adaptive trial, Randomized controlled trial
Abbreviations
- ANSM
Agence nationale de sécurité du médicament et des produits de Santé (French National Agency for Medicines and Health Products Safety)
- COVID
coronavirus disease
- CTA
clinical trial application
- CTCG
clinical trials coordination group
- CTIS
clinical trial information system
- DSMB
data and safety monitoring board
- EC
ethics committee
- EMA
European Medicines Agency
- F-CRIN
French Clinical Research Investigation Network
- FWER
familywise type I error rate
- HAS
Hauteautorité de santé (French National Health Authority)
- HMA
Heads of Medicines Agency
- IA
interim analysis
- PHRC
programme hospitalier de recherche clinique (Clinical Research Hospital Program)
- PROBE
Prospective Randomized Open trial with Blinded Evaluation
- PWER
pairwise type I error rate
- WHO
World Health Organization
Introduction
In recent years, the use of “new methodologies” to assess drug candidates has spread widely, appearing as an alternative to the usual standard of the randomized controlled trial. The objective highlighted for these approaches is to accelerate assessment and to allow patients earlier access to new treatments. However, the term “new methodologies” covers a range of very diverse approaches, some of which, including the use of external controls incorporating data from the care (real-world data) [1], [2], the emulation of a target trial, or the borrowing of information (consisting of enriching the findings of a randomized trial with historical data), have important methodological limitations [3].
Other approaches consist of optimizing the methodological standard of a classic randomized trial, such as trials governed by master protocols (Box 1 ), which rely on a trial design and a single protocol to assess one or more drug candidates in one or more diseases. Among these, platform trials, initially used in the field of oncology [4], [5], have experienced a considerable increase in the assessment of new drug candidates or the repositioning of drugs already marketed for the treatment of coronavirus disease 2019 (COVID-19) [6], [7], [8], [9]. We define as a platform trial a randomized, adaptive trial, potentially without a planned end date, making it possible in a pathology to assess multiple interventions, and which may evolve through the addition or discontinuation of treatment arms according to pre-established rules. The platform trial involves the establishment of an underlying infrastructure making it possible to compare multiple arms, simultaneously or one after the other, to a common control group (Fig. 1 ). The trial is governed by a master protocol that evolves by amendments or additions of successive sub-protocols for each new intervention.
Box 1. Definitions.
Master protocol: a protocol that allows one or more interventions to be assessed within the same clinical trial, in one or more patient populations sharing common enrollment, follow-up, and assessment procedures. Platform, basket, and umbrella trials are different examples of trials for which master protocols are developed.
Platform trial: randomized, adaptive trial, potentially without a scheduled termination date, making it possible in a pathology to assess several interventions, and which may evolve by the addition or discontinuation of treatment arms according to pre-established rules. Platform trials involve the establishment of an underlying infrastructure to compare multiple arms, simultaneously or one after the other, to a common control group. The trial is governed by a master protocol that evolves by amendments or additions of successive sub-protocols for each new intervention.
Basket trial: trial assessing the same treatment in several diseases or disease subtypes. Mainly used in oncology, they may involve a population of patients with cancers affecting different organs but carrying the same alteration or the same molecular profile.
Umbrella trial: a trial assessing different treatments in the same disease characterized by different subtypes potentially sensitive to different treatments; the most common example in oncology is the study of several targeted treatments based on a molecular abnormality in a given tumor type.
Adaptive trial: the term adaptive trial covers a heterogeneous set of experimental designs that have in common, as defined by the FDA, the possibility to make prospectively scheduled alterations to one or more aspects of the experimental design, based on data accumulated during the trial. The adaptive nature of a trial may therefore relate to various objectives, such as recalculating the number of patients to be enrolled in the trial, making a decision on whether or not to continue the trial, adapting the trial population, treatment or dose, or introducing or discontinuing new treatment arms.
Combined trial (or trial “seamless” trial): type of adaptive design where the same study combines a phase 1 trial and a phase 2 trial, or a phase 2 and a phase 3 trial. Patients enrolled in the first phase may also be analyzed in the second if the investigational conditions are identical. They thus avoid the dead times observed between the different phases of the traditional approach and help limit the total number of patients.
Multi-arm, multi-stage (MAMS) trial: an adaptive, randomized, controlled trial assessing multiple treatments simultaneously compared to only one control arm in the same population. These trials include interim analyses, which may focus on an interim endpoint and allow for an early discontinuation of a treatment arm for futility, and a final analysis on a final endpoint to confirm efficacy. The conflation between MAMS trials and platform trials is common, which differentiates them primarily from the no scheduled termination aspect of platform trials.
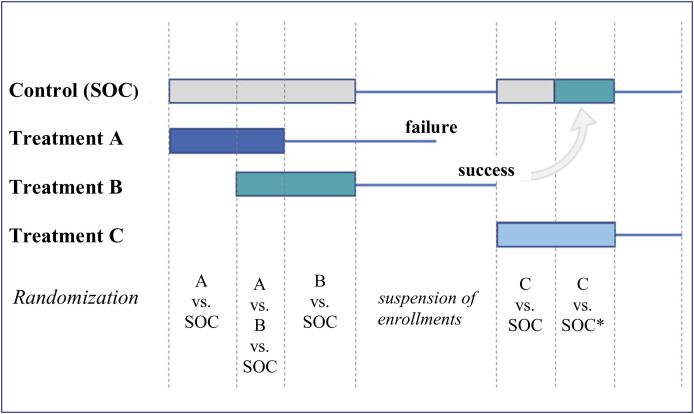
Figure 1.
Schematic representation of the design of a platform trial comparing three new treatments to the standard care (SOC). The rectangles represent the enrollment periods, the fine lines the follow-up periods. The end of follow-up for one arm is reached either at the time of its final analysis or after one of its interim analyses showing efficacy or futility. When the necessary number of subjects is included in an arm, enrollment in the arm is suspended. The new patients are then randomized between the arms that did not reach their headcount and the control arm. When all active arms have reached their expected numbers, enrollment in the study is suspended. SOC* is the new SOC based on treatment B after demonstrating its clinical interest. There is a time limit between the availability of the findings concerning treatment B and its adoption as a new SOC. In the case of treatment C, only patients enrolled during the period corresponding to the new SOC may be used (contemporaneous principle). The first patients enrolled in arm C are not usable, as their contemporaneous control group is no longer loyal at the time of switching to the new SOC.
When should a platform trial be conducted?
The implementation of a platform trial assumes a high potential for recruitment in order to make the initial investment in the infrastructure more profitable. This is useful within the scope of pathologies where there are a lot of drug candidates, whether this involves innovative treatments, repositioning, optimization, or the combination of marketed treatments, or where the standard of care is rapidly changing. Furthermore, this investment is particularly justified for international deployment. Finally, platform trials also prove to be relevant in situations of urgent need for assessment, during which the pooling of protocols and infrastructure accelerates the process, as was the case during the COVID-19 pandemic. Box 2 details some examples of iconic platform trials by their innovative side or their impact.
Box 2. Examples of platform trials.
STAMPEDE: This trial launched in 2005 was initially designed as a multi-arm and multi-stage trial comparing five investigational arms to the standard of care. Other investigational arms have been added over time, while others have been discontinued, making STAMPEDE one of the oldest ongoing platform trials. In total, it has enrolled more than 10,000 patients and compared nine investigational treatments, some of which became the standard of care during the trial.
I-SPY 2: The I-SPY 2 trial is a phase 2 trial with the objective to compare the efficacy of new drug candidates in combination with chemotherapy (standard of care) versus standard of care alone in locally advanced breast cancer. The trial also aims to identify subgroups of responding patients based on molecular characteristics (biomarker signatures) of their disease. The I-SPY 2 trial has totaled 23 experimental treatment arms to date, six of which are still ongoing.
REMAP-CAP: This platform trial comparing different therapeutic strategies, classified by domains (anti-infectives, immunomodulators, antithrombotics), in acute community-acquired pneumonia was designed before the COVID-19 pandemic. However, the trial was fully leveraged to assess a dozen repositioned drugs in the management of COVID-19. Currently still ongoing, the REMAP-CAP trial has enrolled more than 11,000 patients, including approximately 85% with COVID-19.
RECOVERY: The RECOVERY trial is a large platform trial comparing different interventions in COVID-19. This pragmatic trial (including a simplified data collection at most) allowed for the enrollment of nearly 50,000 patients, and has already provided findings for 11 drug candidates, some of which were repositioned.
The potential benefits of a platform trial compared to a standard trial involve:
-
•
establishing an infrastructure including a master protocol, a single sponsor, a stable network of investigator sites with trained teams, a data safety monitoring board (DSMB), a common data processing and pharmacovigilance system, resulting in profitable standardization and economies of scale after an initial investment in the infrastructure;
-
•
the possibility of a shared control arm, which avoids the repeated creation of identical control groups (standard of care) in independent trials, decreasing the number of patients to be enrolled, the costs, and the time limits;
-
•
the possibility of considering the platform trial as a single trial, and of activating each new intervention arm with a simple amendment, which also reduces time limits (and potentially costs).
However, given these potential benefits, the particularity of these trials raises methodological, ethical, and regulatory issues that are presented in this article, as well as the complexity of sponsorship and data management related to the multiplicity of partners, financing, and governance of these trials, and the acceptability of their findings by regulatory registration agencies, and market access agencies. However, we have excluded from this discussion research facilities intended to host several trials conducted in different indications, which essentially facilitate certain logistical aspects.
Methodological issues of platform trials
As with classic randomized trials, it is essential to adhere to the usual methodological principles guaranteeing the robustness of the findings so as not to degrade the reliability of the results. However, the particular design of platform trials exposes us to certain risks that we will detail in this section, by proposing guidance in order to limit them as much as possible.
Enrollment periods for each arm not overlapping
In order to ensure the comparability of groups, comparisons of patients from a group treated with a drug candidate should be made in relation to patients from the control group who are contemporaneous, in order to have equivalent characteristics on average. In the platform trials, the risk of imbalance between groups is increased due to the enrollment periods in the different arms that are not all overlapping (Fig. 1). As a result, the bias related to the non-contemporaneous nature of control patients may be major if there is a rapid evolution in the management of patients. A caricatural example is mortality related to COVID-19 at the beginning of the pandemic. In April 2020, the 30-day mortality of hospitalized patients was on average 12.9%. In May of the same year, it fell to 5.9%. If a new treatment enters a platform trial in May 2020 and includes for the control arm all of the patients who received the usual care (average from April and May 2020 of approximately 9.3%), its use would be associated with a relative reduction of 37% in mortality, without real efficacy [10]. Methods have recently been proposed to correct the analysis in the case of non-contemporaneous controls and show an increase in efficacy that exceeds the risk of bias under certain conditions, but there is a lack of perspective on their use in practice [11].
Guidance #1: for each of the treatment arm comparisons of a platform trial, it is recommended that patients enrolled contemporaneously in the trial be compared.
Multiplicity of comparisons
In a platform trial, several arms are compared to a common control, and arms may be added during the trial. If a non-Bayesian analysis is used, several statistical hypotheses are then tested, raising the issue of multiple testing and control of type one (risk of falsely rejecting the null hypothesis) and type two (risk of not rejecting the null hypothesis under the alternative hypothesis) errors. Three levels of complexity overlap:
-
•
when testing multiple null hypotheses, there are two ways to control the risk of a type-one error: one can seek to control the risk of error of each comparison, also known as the pairwise error rate (PWER) so that each comparison is made at the pre-defined nominal threshold (typically 5%). We can also look to control the probability of falsely rejecting at least one of the multiple hypotheses tested, and we will then talk about the familywise error rate (FWER). In a platform trial, the error we want to control differs depending on the nature of the drug candidate or drugs. If the treatment arms correspond to independent questions, for example different drug candidates, the findings of the test comparing an arm with the control has no reason to influence the other comparisons, which would typically be the subject of separate trials. The rationale selected is to not be stricter in a platform trial than for several independent trials, and we will then seek to control the PWER. On the contrary, if the drug candidates are not independent, for example different doses of the same drug, a test on one arm will have consequences on the other comparisons and the FWER will be controlled instead [12], [13];
-
•
due to the sharing of the control arm, the different tests are correlated. If this correlation does not increase the risk of a type-one error (PWER or FWER), it leads to a difference in relation to the classic situation where different trials would be conducted, increasing the risk of simultaneously concluding by mistake for several comparisons. A specific statistical test approach should then be used. Note that the correlation decreases as the size of the control group increases in relation to the investigational arms;
-
•
finally, the addition of an additional drug candidate arm during the trial theoretically induces an alteration in the PWER and FWER. However, the impact on the PWER is low, and although several statistical methods have been proposed to adjust the level of testing during trials, we do not recommend implementing them.
Guidance #2: a control of the PWER is generally preferred, adjusting the nominal threshold to account for the shared control arm, except in the situation of the same drug candidate tested in several forms (doses, durations, mode of administration, etc.), where the control of the FWER will be preferred.
Interim analyses
Performing interim analyses (IAs) for efficacy and futility is the basis for the platform trial concept. In order to meet the objective of sequencing assessments of new candidates as quickly as possible, it is necessary to have a process that allows one of them to be withdrawn from the trial as soon as the demonstration of its benefit is obtained or, on the contrary, in the case of a suspicion of a very likely absence of interest (futility). All proposed methods to carry out interim analyses for efficacy or futility in a satisfactory manner and at the statistical level (control of the overall alpha risk in particular) are usable in the platform trial (Lan-DeMets function, Bayesian method, etc.). If it could not be prematurely discontinued during the interim analyses, one arm will naturally be terminated at the planned final analysis of the last inferential endpoint (as in a classic trial). Classic reservations that may be raised on the findings of a trial discontinued early persist (particularly overestimation of the effect size, too low duration of follow-up and exposure, etc.).
The platform nature of the trial does not require a particular frequency of IAs. These can be frequent as in the I-SPY-2 trial [4] (which is a phase 2 trial) or follow a more usual design as in the pivotal phase 3 trials (one or two IAs). Nothing in the platform trials objective justifies the carrying out of more IAs than would have been planned in a classic approach. It is also possible to choose not to carry out an efficacy IA.
For each arm, the interim analysis plan should be described in the protocol and statistical analysis plan (their number, the information fractions defining them, the statistical method) as well as the adjoining procedures (independent statistician, DSMB). The completion of the IAs of the different arms, however, poses a logistical problem that can be reduced by trying to synchronize the IAs with identical point dates (snapshots) for all arms. Thus, a given IA may be slightly anticipated or delayed from its theoretical time point set in number of events to coincide with a scheduled time point for the entire trial. The methods for floating IAs (such as Lan-DeMets [14], for which the statistical significance threshold to be achieved depends on the fraction of information available at the time of the IA) are of full interest in this case. Here too, the grouping of several arms in the same field logistics allows us to imagine an economy of means compared to the conducting of separate trials.
Of course, in all cases, safety analyses may be carried out at a frequency that is specific to them.
Guidance #3: although the platform design involves the carrying out of IAs, their de-multiplication at a very high frequency is unnecessary. As in any trial, the design of these interim analyses should be pre-specified.
Implementation of blinding
As with any randomized trial, the conduct of a double-blind platform trial is theoretically feasible and recommended, if we wish to maintain the usual methodological standard of the assessment. Even in some classic trials, the implementation of double-blinding requiring a double placebo (double-dummy) proves to be complex. It is even more important for platform trials where the comparison of multiple treatment arms would require “multiple-dummy” implementation, with all the challenges inherent in this procedure: the loss of flexibility related to the manufacture of placebos, the supply at the different sites, etc.
These logistic burdens explain why the majority of platform trials are conducted in open-label [15]. The platform trial is therefore associated with a risk of degradation of the internal validity of the findings. It is therefore essential to implement measures to best limit biases related to the open-label nature of this type of study, such as the use of “hard clinical criteria” (e.g. mortality), a PROBE (Prospective Randomized Open trial with Blinded Evaluation) design involving an independent and blinded clinical endpoint committee for the treatments, and an intention-to-treat analysis so as not introduce arbitrary post-randomization screening.
Other methodological considerations
As discussed previously, the sharing of the control arm is a source of correlation, which will be lower when the size of the control group increases compared to that of the experimental groups. Some authors recommend a ratio between the control group and k [sic: the] experimental arms for k½. Thus in the STAMPEDE trial which initially included five drug candidates and one control, the allocation ratio was 2:1:1:1:1:1 in favor of it [16].
Other methodological characteristics may be encountered in platform trials, but are not specific to such trials, and are not subject to specific guidance. This is the case for example of Bayesian approaches, relatively common in this situation, or of adaptive randomization, which, for example, has been recommended by the Adaptive Platform Trials Coalition but remains non-consensual to date [17].
Regulatory, ethical, and acceptability aspects of platform trials by registration agencies
Regulatory issue of adding arms
The addition of a new treatment arm in the platform trial may be considered in several ways for regulatory reasons: as an amendment to an existing master protocol (e.g., REMAP-CAP trial [18]), or as a new trial. The first option should be the preferred solution, as it is more flexible and therefore consistent with the philosophy of these designs.
However, the recently implemented European operating framework does not immediately appear suitable to achieve this. Thus, the CTIS implemented under European Regulation 536/2014 on clinical drug trials ensures the handling of coordinated applications for authorization and protocol amendments but does not allow for the management of several simultaneous amendments, which may constitute a limit for platform trials, including multinational trials. In fact, the amendment must be accepted by the ethics committees of the different countries involved in the trial in order to be able to submit a new one. The document produced by the EMA and the HMA/CTCG presents, for complex trials, both options–submission of each intervention arm as a new trial, or submission as an amendment [19]. The submission of each arm as a new trial, however, loses a significant portion of the potential benefits of platform trials, especially as this also implies the establishment of new contracts between the sponsor and the investigator site, new insurance, etc.
As an example, the EU-SolidACT COVID-19 trial (NCT04891133), sponsored by the University of Oslo as part of the EU Response program, had received its initial authorization (master protocol and baricitinib arm) based on a voluntary harmonization procedure (VHP) as part of Directive 2001/20/EC, but the addition of the second arm (bementinib) is done within the framework of European Regulation 536/2014. Due to the impossibility of conducting several simultaneous amendments, the sponsor has chosen to submit it as a new trial, involving the communication to the CTIS of more than 600 documents and the signing of more than 60 agreements with the investigating sites, with no advantage in terms of the authorization time limits.
Guidance #4: ensure that additions of new treatment arms are considered as amendments to an existing master protocol, and not as new trials.
Patient information
Since the platform trial is, as its name indicates, only one and the same trial, the information sheet may be identical for all enrolled patients. It is appropriate from the beginning of the information sheet to explain the adaptive nature of the trial and provide a reminder of it in the consent form. Only those involved in the addition and/or discontinuation of treatment arms, or a change in standard of care (control group), will then be adapted during the trial. The trial sponsor must therefore ensure that the information sheet is updated according to these changes, draft and submit an amendment to the competent authority and the EC, and identify the patients concerned, to avoid signing different versions of consent forms for all patients. An alternative may be to inform and collect the consent in two stages, as was the case in the CORIMUNO trial (NCT04341870): a first consent is required to enter the cohort (which corresponds to standard care, therefore the control group); patients are informed that they can then be randomized to an experimental arm, for which a dedicated information sheet and consent form will be given to the patient.
Guidance #5: the same information sheet for all patients is desirable, with an adaptation of the “treatments” paragraph when adding or removing investigational arms. It is therefore recommended that the sponsor implements robust procedures for tracking changes for the information sheets and patient identification, in order to give the patients the updated versions.
Guidance #6: it is necessary to help ECs understand and master the platform trial design, through information campaigns and carrying out trainings.
Acceptability by competent authorities
At the regulatory agency level, acceptability occurs at three levels: during the application for authorization for a clinical trial protocol, during the application for authorization for the marketing of a new health product or a new indication, and finally during the assessment in view of a reimbursement. In all cases, the methodological aspects remain a priority in the assessment, as well as the guarantee of the safety of the patients/subjects enrolled in these trials.
At the European level, an early scientific opinion is strongly recommended for all sponsors, whether academic or industrial. A joint opinion is even often recommended. This approach is strongly encouraged in a recent publication (in a Q&A format) jointly by the EMA and HMA [19], providing guidance and advice for sponsors or clinical trial managers, whether on scientific aspects, planning, implementation, submission for obtaining a CTA, but also on their use in marketing authorization applications.
In France, the National Health Authority has recently initiated extensive work on “new methodologies” in terms of clinical trials, including platform trials. This reflection follows a referral dated October 4, 2021 by Olivier Véran, then Minister of Solidarity and Health. It aims to develop French methodological and operational expertise in new types of clinical trials, while guaranteeing our country's attractiveness in clinical research. For the HAS, the acceptability of this type of clinical trial is primarily based on compliance with the quality of the methodological principles, in that it does not differ from that of a conventional randomized trial. The HAS has implemented the possibility of early meetings with companies developing pharmaceutical proprietary drugs. These meetings are reserved for products or services deemed innovative due to their mechanism of action and an insufficiently covered medical need, but the innovative nature of the methodology does not justify, on this sole criterion, an early meeting request. Nevertheless, it is strongly encouraged to request a meeting when there is no reference source in the therapeutic area. A joint meeting with pharmaceutical companies and academics carrying out the trial is preferred.
As part of the setting up of a platform trial, it is recommended to have regular exchanges with all stakeholders to avoid any misunderstandings or slowing down in the implementation of this trial. The goal is not to hinder activities that aim to provide patients with an innovative therapeutic solution. The inherent complexity of the platform trial design and the multiplicity of the stakeholders invested, which may have different objectives and constraints depending on the arm, reinforces the need to seek these early opinions.
In addition, sponsors of these trials are advised to follow 8 key principles published in 2019 by the CTCG in order to ensure, in particular, patient safety, scientific integrity, and data integrity [20].
Guidance #7: early contact with agencies, involving all platform trial partners, is recommended prior to the application for trial authorization or in view of assessing the trial findings.
Data usage and communication
Early exchanges must also occur between the various stakeholders (e.g., an academic sponsor and different manufacturers) to define upstream ownership and how to use the data generated in a platform trial. Indeed, the vast majority of platform trials are sponsored by academic teams, but can assess treatments developed by different manufacturers, which are responsible for using the data from these trials as part of the submission of a marketing authorization application dossier.
Guidance #8: anticipate how data will be used between academic sponsors and manufacturers.
Publication of findings by comparison arm and data sharing
If it is required by the regulations to publish and share the findings of a clinical trial upon termination of the study, it is more complex to guarantee this obligation when the trial has no scheduled termination. However, it is critical to guarantee the same level of transparency for platform trials, and for each final analysis of a treatment arm. In its 2019 recommendations, the CTCG encouraged the inclusion in the protocol of a description of the publication policy regarding the interim and final analyses. It is also recommended to specify how to guarantee transparency on data in the trial [20]. For example, for the Inserm-sponsored DisCoVeRy trial, the findings of the first three investigational arms, followed by the fourth arm, were published successively and then updated and confirmed respectively after complete monitoring of the data, a few months later in the same journals [9], [21].
The question is raised in the same terms for sharing trial data: these should be made available to the scientific community in an appropriate registry upon termination of the intervention arm, without waiting for the termination of the platform trial. These rules for the publication and sharing of data are supported by the EMA and HMA [19].
Guidance #9: publish the findings (at a minimum on the CTIS) after the final analysis of each investigational arm, not just upon termination of the platform trial. Data sharing is also recommended.
Funding, sponsorship, organization, governance
Funding
The economic model of a platform trial is strongly linked to its governance mode. A platform trial should be considered an infrastructure capable of addressing multiple scientific questions while declining multiple intervention arms. Therefore, the decision to create (and stop) and finance the platform trial infrastructure should be distinguished from the decision to open a new arm and obtain funding for its completion.
Infrastructure creation and funding
The creation of a platform trial is a complex decision that must take into account the national and international context, avoiding duplication if possible (although ideally the presence of multiple platforms for the same disease, subject to a sufficient potential number of patients, could guarantee the diversity of approaches and avoid monopoly situations).
Consideration should also be given to the ability to gather and coordinate a sufficient network of investigational sites at the national, European, or international level. Where the implementation of a platform trial is relevant (see part 1), it must be one of the objectives of the investigation networks, in order to strengthen their structuring and the coordination of operations in the network. Consideration should also be given to an institution's ability to be a sponsor, with the possible support of (a) co-sponsor(s), the quality of trial and data management, compliance with Good Clinical Practice, and the applicable laws.
This “top-down” rather than “bottom-up” decision mode is similar to decisions regarding the creation of research infrastructures (provide research support and innovation and cover priority societal needs). It should involve scientific advice that can establish priorities in terms of platform creation (and also decide on the potential discontinuation of platform trials), and be supported by funding that makes it possible to implement infrastructure, including the master protocol and if possible, a first intervention arm with its control group. It can also involve partnership funding, such as the EU-PEARL IMI project, which funds development, by public partners and manufacturers for four platform trials (in tuberculosis, depression, neurofibromatosis, and non-alcoholic steatohepatitis). This base funding can be mixed (public-private) and international.
Funding of intervention arms
Funding for each intervention arm may be privately funded when it involves developing innovative products, or public funds when it involves answering a scientific question about repositioning, optimization, or combining approved treatments. This funding can be obtained through calls for classic clinical research projects (Horizon Europe, PHRC, etc.), or through more targeted calls. To ensure the sustainability of the platform, a certain percentile of the private or public financing of the operations of the intervention arm must be allocated to the structuring and common services of the platform trial.
Beyond the funding of intervention arms, there is a question of the relevance of the candidate arms. As a result, an independent mechanism would be necessary to optimize the use of resources mobilizable by the platform trial, especially as they are constrained, even if a candidate arm requests the platform with its funding (see platform access committee, governance chapter).
Guidance #10: distinguish funding from the common infrastructure, which requires initial support, with that of the different treatment arms, which will then contribute to the maintenance of the infrastructure.
Organization and governance
Even more obviously than a classic randomized trial, the governance of a platform trial requires some independence in scientific, ethical, and financial decisions, thanks to the establishment of different committees to decide on different levels of the trial's conduct:
-
•
a Steering Committee, as for any multicenter randomized trial, responsible for the design and implementation of the protocol: choice of investigators, follow-up of enrollments, solutions in case of recruitment difficulties, etc. It will decide with the sponsor on the creation of the platform trial, the management of the infrastructure funding that makes it possible to initiate the trial, as well as on its discontinuation;
-
•
a Data Safety Monitoring Board (or DSMB) that, depending on the missions described in the protocol and the specific charter, ensures the surveillance of the safety, efficacy, or futility of the treatment arms;
-
•
an independent Platform Access Committee (CAP, Comité indépendant d’Accès à la Plateforme), specific to platform trials, which defines the priorities in terms of interventions to be tested and provides guidance to the sponsor and steering committee as to whether or not a new intervention arm should be opened. For the COVID-19 trials funded by the European Commission, this role was performed by the Joint Access Advisory Mechanism (JAAM, https://covid19trials.eu/en/jaam).
Guidance #11: in addition to the committees typically present in multicenter trials, the creation of an independent platform access committee (CAP), whose role is to prioritize the new intervention arms accessing the platform, is recommended.
Sponsorship
Although co-sponsorship has now been possible since the publication of the European Regulation on clinical drug trials (effective on January 31, 2022), the multiplicity of partners makes the organization and coordination of platform trials more complex. Furthermore, as it is difficult to consider for a private stakeholder participating in a platform trial that another private stakeholder is a trial sponsor in which one of its products is assessed, the interest of having only one public structure as the trial sponsor should be emphasized.
Finally, the organizational and logistical complexity of the platform trials involves a detailed distribution of tasks between the different stakeholders, based on homogeneous procedures between the different treatment arms assessed in the trial.
Guidance #12: it is recommended to have only one academic sponsor, with a detailed distribution and delegation of duties.
Conclusion
Platform trials have been experiencing significant growth for a few years, recently amplified by the COVID-19 crisis. While the implementation of these trials in certain situations may optimize the number of patients to be enrolled, the costs, and time limits for carrying out the investigation, they also raise specific issues. From a methodological perspective, platform trials can (and must) provide the same methodological safeguards as classic trials, while respecting, in particular, the contemporaneous nature of the comparisons and while taking into account their multiplicity. Finally, good coordination between all stakeholders and early contact with agencies seems essential to ensure the feasibility of the platform trial, which is heavier than a standard trial regarding the organizational and logistical aspect and to maintain its efficiency.
Disclosure of interest
MR, OD, SL, PB, OC, VD, HE, CF, AGa, LG, CG, AGu, CL, XP, RP, TS, NV declare that they have no competing interest.
MC: The author declares not having any conflicts of interest with private or public entities that have an interest in the design or conducting of these types of studies.
JD: The author declares not having any conflicts of interest with private or public entities that have an interest in the design or conducting of platform trials.
Footnotes
The articles, analyses and proposals resulting from the Giens Workshops are those of the authors and do not prejudge the positions of their organisations.
References
- 1.Cucherat M., Laporte S., Delaitre O., Behier J.M., d’Andon A., Binlich F., et al. From single-arm studies to externally controlled studies. Methodological considerations and guidelines. Therapie. 2020;75:21–27. doi: 10.1016/j.therap.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Patel D., Grimson F., Mihaylova E., Wagner P., Warren J., van Engen A., et al. Use of external comparators for health technology assessment submissions based on single-arm trials. Value Health. 2021;24:1118–1125. doi: 10.1016/j.jval.2021.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Société française de pharmacologie et de thérapeutique . 2022. Acceptabilité des « nouvelles méthodologies » pour l’évaluation des médicaments. Livre blanc des essais Thérapeutiques. https://www.sfpt-fr.org/livreblancmethodo/source/nouvelles%20methodo.pdf. [Accessed 15 November 2022 (118 pp.)] [Google Scholar]
- 4.Barker A., Sigman C., Kelloff G., Hylton N., Da Berry, Esserman L. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86:97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]
- 5.James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Anderson J., et al. STAMPEDE: Systemic therapy for advancing or metastatic prostate cancer — a multi-arm multi-stage randomised controlled trial. Clin Oncol (R Coll Radiol) 2008;20:577–581. doi: 10.1016/j.clon.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 6.LaVange L., Adam S.J., Currier J.S., Higgs E.S., Reineck L.A., Hughes E.A., et al. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV): designing master protocols for evaluation of candidate COVID-19 therapeutics. Ann Intern Med. 2021;174:1293–1300. doi: 10.7326/M21-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Investigators REMAP-CAP., Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Normand S-L.T. The RECOVERY platform. N Engl J Med. 2021;384:757–758. doi: 10.1056/NEJMe2025674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ader F., Peiffer-Smadja N., Poissy J., Bouscambert-Duchamp M., Belhadi D., Diallo A., et al. An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19. Clin Microbiol Infect. 2021;27:1826–1837. doi: 10.1016/j.cmi.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd L.E., Freidlin B., Korn E.L. Platform trials — Beware the noncomparable control group. New Engl J Med. 2021;384:1572–1573. doi: 10.1056/NEJMc2102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roig M.B., Krotka P., Burman C.F., Glimm E., Gold S.M., Hees K., et al. On model-based time trend adjustments in platform trials with non-concurrent controls. BMC Med Res Methodol. 2022;22:228. doi: 10.1186/s12874-022-01683-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collignon O., Gartner C., Haidich A.B., James Hemmings R., Hofner B., Pétavy F., et al. Current statistical considerations and regulatory perspectives on the planning of confirmatory basket, umbrella, and platform trials. Clin Pharmacol Ther. 2020;107:1059–1067. doi: 10.1002/cpt.1804. [DOI] [PubMed] [Google Scholar]
- 13.Choodari-Oskooei B., Bratton D.J., Gannon M.R., Meade A.M., Sydes M.R., Parmar M.K. Adding new experimental arms to randomised clinical trials: Impact on error rates. Clin Trials. 2020;17:273–284. doi: 10.1177/1740774520904346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeMets D.L., Lan K.K. Interim analysis: the alpha spending function approach. Stat Med. 1994;13:1341–1352. doi: 10.1002/sim.4780131308. [discussion 1353-6] [DOI] [PubMed] [Google Scholar]
- 15.Park J.J.H., Siden E., Zoratti M.J., Dron L., Harari O., Singer J., et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. 2019;20:572. doi: 10.1186/s13063-019-3664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmar M.K., Sydes M.R., Cafferty F.H., Choodari-Oskooei B., Langley R.E., Brown L., et al. Testing many treatments within a single protocol over 10 years at MRC CTU at UCL: multi-arm, multi stage platform, umbrella and basket protocols. Clin Trials. 2017;14:451–461. doi: 10.1177/1740774517725697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Adaptive Platform Trials Coalition Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18:797–807. doi: 10.1038/s41573-019-0034-3. [DOI] [PubMed] [Google Scholar]
- 18.Angus D.C., Berry S., Lewis R.J., Al-Beidh F., Arabi Y., van Bentum-Puijk W., et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) study. Rationale and design. Ann Am Thorac Soc. 2020;17:879–891. doi: 10.1513/AnnalsATS.202003-192SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Medicines Agency . 2022. Heads of Medicine Agencies. Complex clinical trials – Questions and answers. https://health.ec.europa.eu/system/files/2022-06/medicinal_qa_complex_clinical-trials_en.pdf. [Accessed 15 November 2022 (29 pp.)] [Google Scholar]
- 20.Heads of Medicines Agencies . 2019. Clinical Trials Facilitation and Coordination Group. Recommendation Paper on the Initiation and Conduct of Complex Clinical Trials. https://www.hma.eu/fileadmin/dateien/Human_Medicines/01-About_HMA/Working_Groups/CTFG/2019_02_CTFG_Recommendation_paper_on_Complex_Clinical_Trials.pdf. [Accessed 15 November 2022 (15 pp.)] [Google Scholar]
- 21.Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Mentré F, Burdet C, DisCoVeRy Study Group Final results of the DisCoVeRy trial of remdesivir for patients admitted to hospital with COVID-19 Lancet. Infect Dis. 2022;22:764–765. doi: 10.1016/S1473-3099(22)00295-X. [DOI] [PMC free article] [PubMed] [Google Scholar]