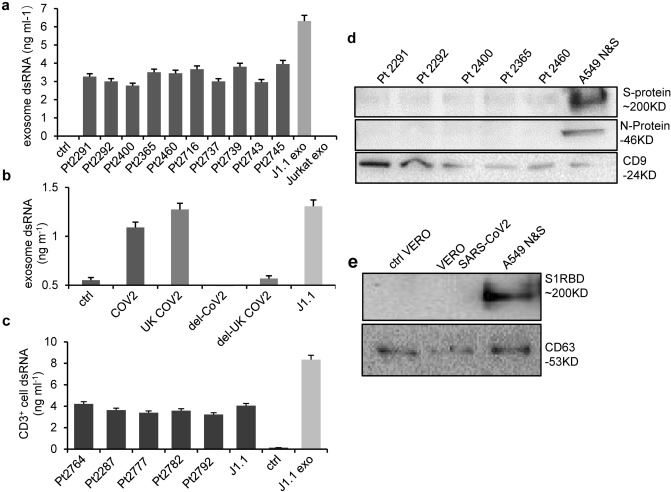
Figure 4.
Viral dsRNA in COVID-19 plasma exosomes and recipient cells. (a) Quantification of dsRNA in COVID-19 plasma exosomes from patients (Pt2291 to Pt2745, average age 49.6) upon hospitalization. J1.1 exo, HIV + J1.1 T-cell exosomes; Jurkat exo, Jurkat T cell exosomes; ctrl, plasma exosomes derived from non-COVID donors. Data represent average ± SD, n = 3. The experiment was repeated 3 times independently. (b) dsRNA quantification in exosomes from control VERO E6 cells (ctrl), SARS-CoV-2-ΔN/EGFP VERO E6 cells (COV), and VERO E6 cells transfected with the UK variant of SARS-CoV-2-ΔN/EGFP (UK COV2). J1.1, J1.1 T-cell exosomes; del-COV2 and del-UK COV2, exosome-depleted cultural supernatants. Data represent average ± SD, n = 3. The experiment was repeated 3 times independently. (c) MicroBeads separated CD3+ lymphocytes from PBMC were treated with COVID-19 plasma exosomes from patients (Pt2764 to Pt2792, average age 61.2) or J1.1 HIV + T-cell exosomes (J1.1 exo) for 30 min, followed by dsRNA measurement in CD3+ lymphocytes. ctrl, non-COVID plasma exosomes. Data represent average ± SD of one experiment out of 3 independent repeats, n = 3. (d) Immunoblot of plasma exosome proteins (100 µg per lane) from COVID-19 patients (Pt2291to Pt2460, average age 42) and (e) A549-hsHA-Nflag cell exosomes overexpressing N and S proteins (A549 N&S). S-protein, SARS-CoV-2 S protein; N-protein, SARS-CoV-2 N protein; S1RBD, S protein blotted using the antibody to S1 receptor binding domain (RBD). CD9 (in d) and CD63 (in e) blots were used as exosome markers on the same blot.