Figure 2.
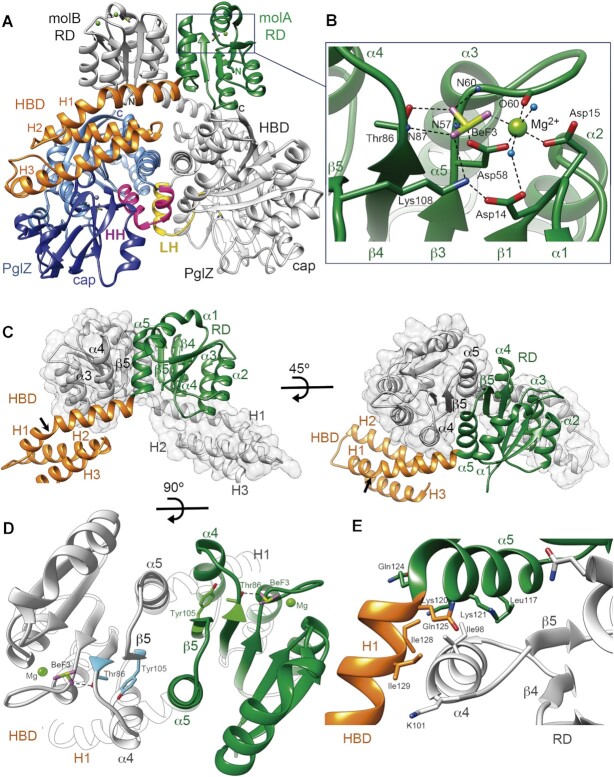
Crystal structure of the PorX dimer. (A) The domains of one PorX monomer (molA) are shown in different colours whereas the entire second monomer (molB) is depicted in grey (except at the contacting LH region). At the N-terminus, the receiver domain (RD, in green) interacts via its α4-β5-α5 surface with the RD of molB, both RDs exposing the active sites at the top of the dimer (BeF3 and Mg2+ are shown as ball and stick models). The following three-helical bundle domain (HBD, in orange) interacts with both the RD of molB and the PglZ domain (in blue) of its own molecule. The PglZ domain contacts the PglZ domain of molB via two helices (HH, in pink) that are folded into a loop and helix in molB (LH, in yellow). The PglZ catalytic and cap subdomains are represented in light and dark blue, respectively. The N- and C-terminal ends in both subunits are indicated by N and C, respectively. (B) Close-up view of the RD active site. The residues that coordinate BeF3 (beryllium and fluorine atoms in pistachio and magenta, respectively) and Mg2+ (light green sphere) are shown as sticks and are labelled. Oxygen and nitrogen atoms are represented in red and blue, respectively. Polar contacts are represented as dashed lines. N59, N60, O60 and N87 correspond to the main-chain atoms of the corresponding residues. Water molecules are represented as light blue spheres. (C) Interface between the two RDs. In one monomer, the RD is represented in green and the HBD in orange, the other monomer is depicted in grey. The RD is followed by the HBD, whose α-helices are indicated (H1, H2 and H3). The arrow shows the kink in H1. (D) RD dimer from the top. The interface helices α4 and α5 and β-strand 5 (β5) are indicated. Tyr105 and Thr86 (in blue and green in molA and molB, respectively), BeF3 and Mg2+ are depicted. (E) Sharp bend between RD helix α5 and H1 from the HBD. The hydrophobic core that stabilizes the inter-subunit interaction involves the burial of molB Ile98 (helix α4) into the hydrophobic cavity built by molA Leu117, Lys120 and Lys121 methylene side chains, Gln124 from helix α5, and Gln125, Ile128 and Ile129 from helix H1. (D) and (E) use the same colour code as in (C).